C1 - Particles
Atoms and Particles
States of Matter
Though the particle model is not perfect, as in reality particles are compounds, atoms and ions. Furthermore, it does not take into account the forces of attraction between particles, or the space between them. However, models like this are still useful as they help us visualise and understand complicated concepts.
History of the Atom
✏✏
C2 - Elements, Compounds and Mixtures
The Periodic Table
In 1869, Dimitri Mendeleev created the periodic table.
C3 - Chemical Reactions
Conservation of Mass
The law of conservation of mass states that for any closed system of mass and energy, the mass of the system will remain constant over time. This means that during a chemical reaction, no atoms are created and no atoms are destroyed.
For example during the combustion reaction of methane, the total mass and number of atoms are the same after the reaction as before the reaction.
\[\ce{CH4 + 2O2 -> CO2 + 2H2O}\]4 atoms of hydrogen, 4 atoms of oxygen and 1 atom of carbon are present before and after the reaction.
However, when conducting chemical reactions, sometimes it appears that mass has been lost, however, this is usually due to there being a gas involved. For example the reaction could have gas escaping into the atmosphere, or use gas from the atmosphere as part of the reaction.
Chemical Equation Notation
Word Equations
Word equations show a chemical reaction through words which make them easier to understand. These show the reactants on the left side and the products on the right side.
\[\text{ methane } + \text{ oxygen }\ce{ -> } \text{ carbon dioxide } + \text{ water }\]Symbol Equations
Symbol equations use atomic symbols to show the formulae of the reactants and products, symbol equations must always been balanced, meaning the number of atoms on both side of the equation must be the same.
For example the symbol equation for the reaction of sulfuric acid with sodium hydroxide can be denoted as
\[\ce{H2SO4 + NaOH -> Na2SO4 + H2O}\]State Symbols
State symbols are used to notate the physical state the reactants and products are in.
| State Symbol | Meaning |
|---|---|
| $\ce{s}$ | Solid |
| $\ce{l}$ | liquid |
| $\ce{g}$ | gas |
| $\ce{aq}$ | aqueous |
For example, aqueous hydrochloric acid reacts with solid calcium carbonate to form aqueous calcium chloride, liquid water and carbon dioxide gas.
\[\ce{2HCl_{(aq)} + CaCO_{3(s)} -> CaCl_{2(aq)} + H2O_{(l)} + CO_{2(g)}}\]Ionic Equations
Ionic equations can be written for any reaction that involves ions in a solution. AN ionic equation only includes reacting particles.
\[\ce{CaCl_{2(aq)} + 2NaOH_{(aq)} -> Ca(OH)_{2(s)} + 2NaCl_{(aq)}}\]In order to create an ionic equation, we must take the reaction equation and break aqueous substances into their ions.
\[\ce{Ca^{2+}_{(aq)} + 2Cl-_{(aq)} + 2Na+_{(aq)} + 2OH-_{(aq)} -> Ca(OH)_{2(s)} + 2Na+_{(aq)} + 2Cl-_{(aq)}}\]Then we can cancel out the substances that remain the same on both sides to give us the ionic equation.
\[\ce{Ca^{2+}_{(aq)} + 2OH-_{(aq)} -> Ca(OH)_{2(s)} }\]Half Equations
Half equations show the movement of electrons during reactions, in half equations $\ce{e-}$ stands for one electron. Half equations can be written for reactions where oxidisation or reduction happens.
For example, the half equations for the reduction of hydrogen can be displayed as
\[\ce{2H+ + 2e- -> H2}\]Half equations can also be combined to form full ionic equations, for example the two following half equations
\[\ce{Na -> Na+ + e-}\\ \ce{2H+ + 2e- -> H2}\]Can be combined to form
\[\ce{2Na + 2H+ -> 2Na+ + H2}\]Full ionic equations should never contain electrons as the electrons in the reactants and products should cancel out.
Moles
Moles are a unit of measurement used to measure the amount of a substance. It can be defined as exactly $6.02214076×10^{23}$ entities, which is the amount of particles in 1 gram of substance.
This means that $1 \mathrm{mol}$ of a substance is always equal to its relative atomic mass($A_r$) or relative formula mass($M_r$) in grams. For example:
-
Carbon has an $A_r$ of $12$. This means that $1\mathrm{mol}$ of carbon has a mass of exactly $12\mathrm{g}$.
-
Nitrogen gas ($\ce{N2}$) has an $M_r$ of $2\times14=28$. This means that $1 \mathrm{mol}$ of nitrogen gas has a mass of exactly $28\mathrm{g}$.
-
Hexane ($\ce{C6H14}$) has an $M_r$ of $(6\times 12)+(14\times 1) = 86$. This means that $1\mathrm{mol}$ of hexane has a mass of exactly $86\mathrm{g}$.
This means that to find the number of moles in a substance, we can use the following formula
\[n = \frac{m}{M_r}\]$n$ = number of moles
$m$ = mass of substance
$M_4$ = relative formula mass
Endothermic Reactions
C4 - Alkali Metals
Properties of Elements
Alkali Metals
The metals in group 1 are known as alkali metals, this is because they react to form alkali with water. Alkali metals include: Lithium($\ce{Li}$), Sodium($\ce{Na}$), Potassium($\ce{K}$), Rubidium($\ce{Rb}$) and Francium($\ce{Fr}$).
Alkali metals do not behave in the way one would expect metals to behave, and have low melting and boiling points, low density and are very soft. Furthermore, they are highly reactive as they contain only 1 electron, making it easy to lose to a reaction.
Alkali Metals become more reactive as we go down the group, this is because the singular electrons are further away from the nucleus, making them easier to lose to reactions as we go down the group.
Ionic compounds
Because alkali metals lose their singular electron so easily, they commonly form ionic compounds, for example, sodium chloride is formed when a sodium atom gives its electron to a chlorine atom, making them opposite ions, thereby attracting and forming an ionic compound.
Reaction with cold water
Alkali metals react vigorously in water, and this reaction becomes more vigorous the lower we go in the periodic table. These reactions produce a hydroxide(alkali) and hydrogen gas.
For example, reacting sodium with water produces hydrogen gas and sodium hydroxide.
\[\ce{2Na + 2H2O -> 2NaOH + H2}\]Halogens
Elements in group 7 are known as halogens, this includes the elements: fluorine($\ce{F}$), Chlorine($\ce{Cl}$), Bromine($\ce{Br}$), iodine($\ce{I}$) and astatine($\ce{At}$).
All halogens exist as diatomic molecules, forming covalent bonds as they share 1 electron with each other, $e.g. \ce{Cl2, Br2, I2}$.
As we go down group 7, melting points and boiling points increase, while reactivity decreases. This is because as we go down the group, the outer shell becomes further and further away from the nucleus, lessening the attractive force of the nucleus and making it harder to pull in an electron to complete the full shell.
At room temperature, Chlorine($\ce{Cl2}$) exists as a fairly reactive, poisonous and green gas; Bromine($\ce{Br2}$) as a poisonous, red-brown liquid and Iodine($\ce{I2}$) as a dark grey crystalline solid that releases purple vapour when heated.
Reaction with Alkali Metals
Halogens easily form ionic compounds with the aforementioned alkali metals, this is because alkali metals easily give an electron to form a full shell, while halogens easily take an electron to form a full shell. After the electron transaction, both ions become attracted to each other and form an ionic bond.
For example, sodium can react with chlorine gas to form sodium chloride, also known as table salt.
\[\ce{2Na + Cl2 -> 2NaCl}\]Displacement Reactions
More reactive halogens can displace less reactive halogens from a salt solution in what is known as a displacement reaction.
For example, chlorine is more reactive than bromine and so can displace it in a displacement reaction.
\[\ce{Cl_{2(g)} + 2KBr_{(aq)} -> Br_{2(g)} + 2KCl_{(aq)}}\]Displacement reactions can be used to show reactivity trends as solutions often change appearance when a displacement reaction occurs.
Noble Gases
Elements in group 0 are known as noble gases. This includes elements such as: helium($\ce{He}$), neon($\ce{Ne}$), argon($\ce{Ar}$), krypton($\ce{Kr}$) and xenon($\ce{Xe}$).
Noble gases are monoatomic, which means their gases are made out of singular atoms, this is because they are inert(stable) and do not react with other elements as they have a full shell of electrons.
This makes them non-flammable and makes them hard to observe.
As we go down the group, boiling point, melting point, and density all increase.
Transition Metals
Transition metals are metals with typical metallic properties that do not fall in any of the groups. Properties of transition metals including being hard, strong and shiny.
Most transition metals also have high melting points and high densities.
Transition metals and the compounds they form make extremely good catalysts, for example, iron is the catalyst used in the Haber process.
Furthermore, transition metals often have more than one ion, for example, copper can be found as $\ce{Cu+}$ and $\ce{Cu^{2+}}$.
Transition metals often form different colours in compounds.
Transition metals are also relatively unreactive compared to group 1 and group 2 elements but will react with dilute acids to form metal salts.
Reactivity
C5 - Monitoring and Controlling Chemical Reactions
Concentration
Concentration is the amount of substance
C6 - Global Challenges
Extracting Metals
Reduction through Carbon
Metal ores are normally found as oxides of the metal, and so have to have the pure metal extracted. For example, the main aluminium ore is called bauxite, which is the name for aluminium oxide ($\ce{Al2O3}$).
Oxidisation is the reaction of a substance with oxygen, reduction is the process of removing the oxygen.
\[\ce{Fe2O3 + 3CO ->2Fe +3CO2}\]Iron oxide is reduced to iron, and carbon monoxide is oxidised to carbon dioxide.
Metals can be extracted using reduction using carbon, which removes the oxygen from it. For example, copper oxide can be reduced with carbon to pure copper:
\[\ce{2CuO + C -> 2Cu + CO2}\]However, not all metals can be extracted with carbon, as if carbon is less reactive than a metal, a reaction will not occur. To determine weather a metal can be reduced with carbon, the reactivity series is used.
\[\underline{\text{The Reactivity Series}}\\ \qquad \qquad \qquad \qquad \qquad \qquad\small{\text{ more reactive }}\big\uparrow\\ \begin{aligned} &\text{Potassium} &\ce{K}\\ &\text{Sodium} &\ce{Na}\\ &\text{Calcium} &\ce{Ca}\\ &\text{Magnesium} &\ce{Mg}\\ &\text{Aluminium} &\ce{Al}\\ &\textbf{Carbon} &\textbf{C}\\ &\text{Zinc} &\ce{Zn}\\ &\text{Iron} &\ce{Fe}\\ &\text{Tin} &\ce{Sn}\\ &\text{Copper} &\ce{Cu}\\ \end{aligned}\]For more reactive metals, electrolysis of molten compounds has to be used, which is much more expensive than reduction with carbon.
Extracting Iron ore
As Iron is less reactive than carbon, it can be extracted from its ore using reduction by carbon. Extracting iron ore in industry is done with three raw materials, iron ore, coke(carbon) and limestone(calcium carbonate - $\ce{CaCO3}$).
This reaction takes place in a blast furnace, where the reaction chamber is kept by hot jets of air.
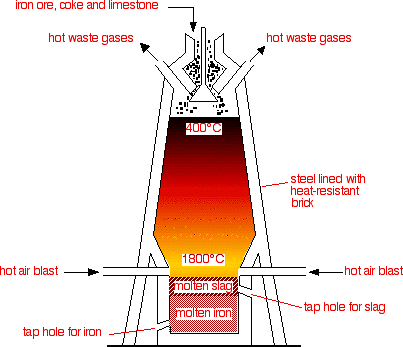
Blast furnace. From: Chemguide
First, coke is burnt in hot air, creating carbon dioxide,
\[\ce{C_{(s)} +O_{2(g)} -> CO_{2(g)}} \tag{step 1}\]More coke reduces the $\ce{CO2}$, creating carbon monoxide,
\[\ce{C_{(s)} + CO_{2(g)} -> 2CO_{(g)}} \tag{step 2}\]At $1500°\mathrm{C}$, carbon monoxide reduces iron(III) oxide to pure iron, this causes the molten iron to sink to the bottom of the furnace, where it can be extracted.
\[\ce{Fe2O3 + 3CO_{(g)} -> 2Fe_{(l)} + 3CO_{2(g)}} \tag{step 3}\]Finally, to remove impurities caused by sand, calcium oxide that decomposed from calcium carbonate can be reacted with silica(sand) to form calcium silicate also known as slag which floats on top of the molten iron.
\[\ce{CaCO3 -> CaO + CO2}\\ \ce{CaO + SiO2 -> CaSiO3}\]Biologically extracting Metals
Bioleaching
Bioleaching is the process of extracting metals from their ores using living organisms. This process can use bacteria to break down metals, but often produces toxic substances that can harm the environment.
However, bioleaching is generally considered simpler and cheaper than traditional processes.
Phytoextraction
As the Earth’s supply of metal ores is limited, other methods such as phytoextraction have been conceived as a viable option to extract low-grade ores using living organisms.
This works by using plants to absorb mineral ions through their roots and then harvesting and burning the plant, which leaves behind metal compounds in the ash. Although this process is extremely slow, it reduces the need to obtain new ore by mining and reduces the amount of rock waste created by traditional mining.
Purifying Copper with Electrolysis
Although copper can be easily extracted by reduction with carbon, by smelting, this results in impure copper that does not conduct electricity very well. This is not very useful because a lot of copper is used to make electrical wiring.
This is why electrolysis is used to purify it, even though it is expensive. This process can be shown using the half equation,
\[\ce{Cu -> Cu^2+ +2e-}\\[4pt] \ce{Cu^2+ + 2e -> Cu}\]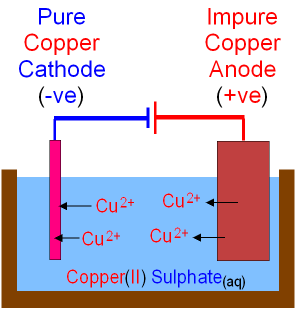
Purification of copper by electrolysis By: GCSEscience.com
Alloys and Corrosion
Properties of Alloys
Alloys are mixtures of two or more different metals. They can also be a mixture of a metal and a non-metal like steel. Alloys often have different properties from the metals they are made from which allows them to be more useful in certain situations.
Steel
Steel is an alloy of iron and carbon. There are several advantages to using steel as it has unique properties, such as:
-
Steel is harder than iron.
-
Steel is stronger than iron.
-
Iron by itself will rust, however steel is less likely to rust.
Many things are made from steel including bridges, engine parts, saucepans, etc.
Brass
Brass is an alloy of copper and zinc. Brass is:
- Harder than copper and zinc.
Brass is useful for making musical instruments, it is also used for fixtures and fittings, like screws and springs.
Bronze
Bronze is an alloy of copper and tin. It is:
-
Harder and stronger than tin.
-
More resistant to corrosion than copper and tin.
Bronze is used to make springs and motor bearings. It is also used to make bells and widely used in sculpture.
Solder
Solder is usually an alloy of lead and tin. Solder does not have a definite melting point, but instead gradually cools. This makes it useful for joining metals together and can be used to connect components in electrical circuits.
Duralumin
Duralumin is a compound of aluminium, copper and magnesium. It is:
-
Very low density.
-
Stronger than aluminium.
Because it is strong and light, it can be used for making parts of aeroplanes and other aircraft.
Corrosion
Corrosion is the process where a metal is slowly damaged by chemically reacting with substances in the environment.
An example of this is rusting, which is where iron reacts with oxygen and water from the environment, to form hydrated iron(III) oxide(rust).
\[\ce{4Fe + 3O2 + 6H2O -> 4Fe(OH)3}\\ \small\ce{iron + oxygen + water -> hydrated}\text{ iron(III) oxide}\]Rusting is a redox reaction as each iron atom loses three electrons to become $\ce{Fe^3+}$, which means that iron is oxidised. Each oxygen atom gains two electrons to become $\ce{O^2-}$, which means that oxygen is reduced.
This means that this redox reaction can be written with the half equations,
\[\ce{Fe -> Fe^2+ + 2e-}\\[5pt] \textstyle{\frac{1}{2}}\ce{O_2 + 2e- + H2O -> 2OH-}\\[5pt] \ce{2Fe^2+ + }\textstyle{\frac{1}{2}}\ce{O2 -> 2Fe^3+ + O^2-}\]Preventing Corrosion
Oil, grease and paint can prevent corrosion. This is because they can keep out water, oxygen or both. Painting is better for large structures, whilst oil and grease are more effecting where moving parts are involved, like bicycle chains.
Furthermore, tin plating can be used to prevent steel from corroding. Tin can act as a barrier against water and oxygen. However, if the tin coating is scratched or damaged, the steel will corrode.
Sacrificial methods can also be used, where a more reactive metal is placed with the metal that needs to be protected, water and oxygen will react with the metal with higher reactivity, thereby protecting the metal.
Industrial Processes
The Haber Process
The Haber process is one of the most important industrial processes in the world, as the ammonia it produces is used to make nitrogen based fertilisers.
\[\ce{N2_{(g)} + 3H2_{(g)} <=> 2NH3_{(g)} (+ heat)}\]To make ammonia on a large scale, a lot of nitrogen and hydrogen is needed.
Nitrogen can be easily obtained from the air, which is composed of $70\%$ nitrogen. The hydrogen is more difficult to obtain and can be produced from hydrocarbons from natural gases or hydrocarbons.
Because the reaction is reversible, the ammonia breaks back down into nitrogen and hydrogen once the reactions reaches equilibrium.
The Haber Process on an industrial scale
In industry, the Haber process utilises large chambers that are fed $\ce{H2}$ and $\ce{N2}$, which creates ammonia. This ammonia gas is cooled in a condenser chamber, where it becomes a liquid. However, because ammonia has a lower boiling point than hydrogen and nitrogen, the hydrogen and nitrogen do not become liquids, and are recycled into the main reactor.
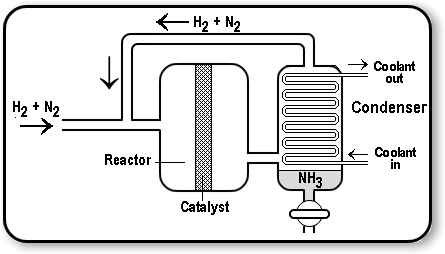
The Häber Process. From: Chemstuff
The iron catalyst does not increase yield or affect he position of equilibrium, however, it speeds up the amount of time taken to reach the equilibrium position.
Finally, in industry, ammonia is created at a pressure of $200 \mathrm{atm}$ and $450°\textrm{C}$.
This is because the reaction is exothermic, and releases heat, so a lower temperature is needed to achieve higher % yield. However, a lower temperature reduces the rate of reaction. Therefore, a compromise is made at $450°\textrm{C}$, to ensure the maximum possible yield.
Pressure is kept at $200 \mathrm{atm}$ because to achieve higher percentage yield, higher pressure is needed. as there are more molecules of products as there are of reactants. High pressure also achieve higher rate of reaction. However, pressure cannot be too high, as very high pressures can be both expensive and dangerous. This is why $200\mathrm{atm}$ is used to achieve best yield : cost ratio.
Fertilisers
The three main elements in fertilisers are nitrogen, phosphorus and potassium. Which is why they are sometimes called $\ce{NPK}$ fertilisers. These elements are crucial to plant growth, and can be placed in soil to replace missing soil nutrition, or to increase soil nutrition to provide for a greater crop yield, so that crops will grow faster and bigger.
Ammonia is a base and it can be neutralised with acids to make ammonium salts, which are used commonly in soil fertilisers.
Ammonium nitrate can be made by neutralising ammonia with nitric acid,
\[\ce{HNO3 + NH3 -> NH4NO3}\]Ammonium sulphate can be made by neutralising ammonia with sulfuric acid,
\[\ce{2NH3 + H2SO4 -> (NH4)2SO4}\]Ammonium phosphate can be made by neutralising ammonia and phosphoric acid,
\[\ce{H3PO4 + 3NH3 -> (NH4)3PO4}\]Finally, potassium nitrate is also a fertiliser, and can be made by neutralising nitric acid with potassium hydroxide.
\[\ce{KOH + HNO3 -> KNO3 + H2O}\]The Contact Process
The contact process is used to make sulfuric acid.
The first stage is to make sulphur dioxide by burning sulphur in air.
\[\ce{S_{(s)} + O_{2(g)} -> SO_{2(g)}} \tag{stage 1}\]Then, the sulphur dioxide is oxidised to make sulphur trioxide.
\[\ce{2SO_{2(g)} + O_{2(g)} <=> 2SO_{3(g)}} \tag{stage 2}\]Finally, the sulphur trioxide is used to make sulfuric acid.
\[\ce{SO_{3(g)} + H2O_{(l)} -> H2SO_{4(aq)} } \tag{stage 3}\]The Contact Process on an Industrial Scale
The reaction in stage 2 is reversible. So reaction conditions have to be carefully controlled to secure the best equilibrium position.
Temperature
-
Oxidising sulphur dioxide to form sulphur trioxide is exothermic.
-
So to get higher yield, temperature has to be reduced.
-
However, reducing the temperature slows the rate of reaction.
-
So a compromise temperature of of $450°\mathrm{C}$ is used.
Pressure
-
There are two moles of gas on the product side, while there are three moles of gas on the reactants side.
-
So to get a higher yield, pressure should be increased.
-
However, increase pressure is expensive, and equilibrium already lies to the right.
-
Therefore, the reaction is typically carried out at $1 \mathrm{atm}$ or just above
Catalyst
-
To increase the rate of reaction vanadium pentoxide is used as a catalyst.
-
This does not change the position of equilibrium.
Materials
Life-Cycle Assessments
A life-cycle assessment look at each stage in a products life cycle and works out the potential environmental impact of each stage.
Extracting and Processing Raw Materials
Extracting raw materials can damage the local environment by doing things like destroying natural habitats e.g. cutting down forests.
Processing raw materials can also indirectly damage the environment as they require huge amounts of energy and may release large amounts of pollutants.
Manufacture and Packaging
Manufacturing products uses a lot of energy and other resources. Manufacture can also release pollution, like harmful gases such as $\ce{CO}$ or $\ce{HCL}$. Furthermore, manufacture can also release waste products and pollute water.
Product Use
Depending on how long a product is used and other factors, product use can damage the environment. For example, paint releases toxic fumes, and fertilisers can leach into streams and rivers and cause damage to ecosystems.
Disposal
Products are often disposed in a landfill site. This takes up space and can pollute land and water. Products can also be incinerated, which causes air pollution.
Recycling Materials
✏✏✏✏✏✏
Homologous Series
A homologous series is a group of chemicals that have the same functional group and similar chemical properties.
Generally, chemicals in a homologous series follow a common naming convention with standard prefixes and differing suffixes for each different series:
| Prefix | Alkanes(-ane) | Alkenes(-ene) | Alcohols(-anol) |
|---|---|---|---|
| Meth- | Methane | - | Methanol |
| Eth- | Ethane | Ethene | Ethanol |
| Prop- | Propane | Propene | Propanol |
| But- | Butane | Butene | Butanol |
| Pent- | Pentane | Pentene | Pentanol |
| Hex- | Hexane | Hexene | Hexanol |
Note that there is no “methene” as alkenes are unsaturated hydrocarbons which means at least 2 carbons are needed to form a double bond.
The term saturated refers to homologous groups who’s chemical’s only contain $\ce{C-C}$ bonds between carbon atoms.
A functional group is a section of a hydrocarbon that defines how the chemical reacts, members of the same homologous series all contain the same functional group.
Alkanes
Alkanes are a homologous series of hydrocarbons, each alkane in the series increases by 1 carbon and 2 hydrogen atoms, and so the general formula for alkanes can be denoted as:
\[{\ce{C}_n}{\ce{H}_{2n + 2}}\]Where $n$ stands for the number of carbon atoms.
Using this formula, we can derive the formula of different alkanes:
\[\begin{aligned} &\small{\negthinspace\quad\enspace\ce{H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\text{ }\quad}\\[-5pt] &\small{\ce{H\bond{-}C\bond{-}H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\text{ }\quad}\\[-5pt] &\small{\negthinspace\quad\enspace\ce{H}}\\[4pt] \text{Me}&\text{thane}\ce{(CH4)} \end{aligned} \qquad \begin{aligned} &\small{\negthinspace\quad\enspace\ce{H}\negthinspace\quad\negthinspace\negthinspace\thinspace\ce{H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|}\\[-5pt] &\small{\ce{H\bond{-}C\bond{-}C\bond{-}H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|}\\[-5pt] &\small{\negthinspace\quad\enspace\ce{H}\negthinspace\quad\negthinspace\negthinspace\thinspace\ce{H}}\\[4pt] &\text{Ethane}\ce{(C2H6)} \end{aligned} \qquad \begin{aligned} &\small{\negthinspace\quad\enspace\ce{H}\negthinspace\quad\negthinspace\negthinspace\thinspace\ce{H}\negthinspace\quad\negthinspace\negthinspace\thinspace\ce{H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|\negthinspace\text{ }\quad|}\\[-5pt] &\small{\ce{H\bond{-}C\bond{-}C\bond{-}C\bond{-}H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|\negthinspace\text{ }\quad|}\\[-5pt] &\small{\negthinspace\quad\enspace\ce{H}\negthinspace\quad\negthinspace\negthinspace\thinspace\ce{H}\negthinspace\quad\negthinspace\negthinspace\thinspace\ce{H}}\\[4pt] &\negthinspace\text{Propane}\ce{(C3H8)} \end{aligned} \qquad \begin{aligned} &\small{\negthinspace\quad\enspace\ce{H}\negthinspace\quad\negthinspace\negthinspace\thinspace\ce{H}\negthinspace\quad\negthinspace\negthinspace\thinspace\ce{H}\negthinspace\quad\negthinspace\negthinspace\thinspace\ce{H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|\negthinspace\text{ }\quad|\negthinspace\text{ }\quad|}\\[-5pt] &\small{\ce{H\bond{-}C\bond{-}C\bond{-}C\bond{-}C\bond{-}H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|\negthinspace\text{ }\quad|\negthinspace\text{ }\quad|}\\[-5pt] &\small{\negthinspace\quad\enspace\ce{H}\negthinspace\quad\negthinspace\negthinspace\thinspace\ce{H}\negthinspace\quad\negthinspace\negthinspace\thinspace\ce{H}\negthinspace\quad\negthinspace\negthinspace\thinspace\ce{H}}\\[4pt] &\space\space\text{Butane}\ce{(C4H10)} \end{aligned}\]Although all alkanes have similar properties, there are many trends in property change as the chain increases.
As $n$ increases and the alkane increases in length, boiling points increase, chemicals such as methane($\ce{CH4}$ ) and ethane($\ce{C2H6}$) have low boiling points and so are gases at room temperature. However, longer alkanes with more than 4 carbons are liquid at room temperature. Alkanes with really long chains such as octadecane($\ce{C18H38}$) are solid at room temperature.
In addition, shorter alkanes are more flammable which makes them useful for fuel.
Combustion reactions
Alkanes can take part in complete combustion, where they are oxidised and release huge amounts of energy, making it a exothermic reaction.
\[\ce{hydrocarbon + oxygen -> carbon dioxide + water}\]For example, the complete combustion of ethane is denoted as:
\[\ce{C2H6 + 3\negthinspace\tfrac{1}{2}O2 -> 2CO2 + 3H2O}\]However, when there is a limited supply of oxygen, incomplete combustion occurs, where carbon monoxide is produced.
\[\ce{hydrocarbon + oxygen -> carbon monoxide + water}\]For example, the incomplete combustion of ethane is denoted as:
\[\ce{C2H6 + 2\negthinspace\tfrac{1}{2}O2 -> 2CO + 3H2O}\]Note that both complete and incomplete combustion can occur at the same time in a mixture, giving a mixture of combustion products.
Alkenes
Alkenes are a homologous series of hydrocarbons that have a functional group of $\ce{C=C}$, making them unsaturated as they contain a double bond in their functional group. Alkenes all have double the number of hydrogen to carbon, and can be denoted with the general formula:
\[\ce{C}_n \ce{H}_2n\]Where $n$ stands for the number of carbon atoms.
Using this formula, we can derive the formula of different alkenes:
\[\begin{aligned} \small{\ce{H}}&\quad\quad\quad\quad\small{\ce{H}}\\[-8pt] &\small{\diagdown\negthinspace\enspace\enspace\enspace\text{ }\enspace\space\text{ }\diagup}\\[-8pt] &\small{\quad\ce{C\bond{=}C}}\\[-8pt] &\small{\diagup\negthinspace\enspace\enspace\enspace\text{ }\enspace\space\text{ }\diagdown}\\[-8pt] \small{\ce{H}}&\quad\quad\quad\quad\small{\ce{H}}\\[4pt] \text{E}&\text{thene}\ce{(C2H4)} \end{aligned} \qquad \begin{aligned} &\small{\negthinspace\quad\enspace\ce{H}\space\negthinspace\negthinspace\thinspace\negthinspace\quad\negthinspace\negthinspace\ce{H}\thinspace\qquad\ce{H}}\\[-8pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|\enspace\quad\diagup}\\[-8pt] &\small{\ce{H\bond{-}C\bond{-}C\bond{=}C}}\\[-8pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\quad\qquad\diagdown}\\[-8pt] &\small{\negthinspace\quad\enspace\ce{H}\qquad\quad\enspace\negthinspace\ce{H}}\\[4pt] &\text{Propene}\ce{(C3H6)} \end{aligned} \qquad \begin{aligned} &\small{\negthinspace\quad\enspace\ce{H}\space\negthinspace\negthinspace\thinspace\negthinspace\quad\negthinspace\negthinspace\ce{H}\space\negthinspace\negthinspace\thinspace\negthinspace\quad\negthinspace\negthinspace\ce{H}\thinspace\qquad\ce{H}}\\[-8pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|\negthinspace\text{ }\quad|\quad\enspace\diagup}\\[-8pt] &\small{\ce{H\bond{-}C\bond{-}C\bond{-}C\bond{=}C}}\\[-8pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|\enspace\enspace\negthinspace\qquad\diagdown}\\[-8pt] &\small{\negthinspace\quad\enspace\ce{H}\space\negthinspace\negthinspace\thinspace\negthinspace\quad\negthinspace\negthinspace\ce{H}\qquad\quad\enspace\ce{H}}\\[4pt] &\enspace\text{ Butene}\ce{(C4H8)} \end{aligned} \qquad \begin{aligned} &\small{\negthinspace\quad\enspace\ce{H}\space\negthinspace\negthinspace\thinspace\negthinspace\quad\negthinspace\negthinspace\ce{H}\space\negthinspace\negthinspace\thinspace\negthinspace\quad\negthinspace\negthinspace\ce{H}\space\negthinspace\negthinspace\thinspace\negthinspace\quad\negthinspace\negthinspace\ce{H}\enspace\thinspace\qquad\ce{H}}\\[-8pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|\negthinspace\text{ }\quad|\negthinspace\text{ }\quad|\thinspace\qquad\diagup}\\[-8pt] &\small{\ce{H\bond{-}C\bond{-}C\bond{-}C\bond{-}C\bond{=}C}}\\[-8pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|\negthinspace\text{ }\quad|\negthinspace\text{ }\quad|\enspace\negthinspace\qquad\diagdown}\\[-8pt] &\small{\negthinspace\quad\enspace\ce{H}\space\negthinspace\negthinspace\thinspace\negthinspace\quad\negthinspace\negthinspace\ce{H}\space\negthinspace\negthinspace\thinspace\negthinspace\quad\negthinspace\negthinspace\ce{H}\space\negthinspace\negthinspace\thinspace\negthinspace\quad\negthinspace\negthinspace\ce{H}\qquad\enspace\enspace\ce{H}}\\[4pt] &\enspace\text{ Pentene}\ce{(C5H10)} \end{aligned}\]Testing for alkenes
The presence of the $\ce{C=C}$ double bond means that alkenes react differently to alkanes, the two chemicals can be differentiated with a simple test.
This can be done with bromine water($\ce{Br2}$), which is orange in colour, the alkene will decolourise the bromine water, turning it from orange to colourless. For example, reacting ethene($\ce{C2H4}$) with bromine($\ce{Br2}$) makes dibromoethane($\ce{C2H4Br2}$), which is colourless:
\[\ce{C2H4 + Br2 -> C2H4Br2}\]This is because the double bond can be opened up and form bonds with bromine, which cannot be done with alkanes which are saturated.
\[\begin{aligned} &\begin{aligned} \small{\ce{H}}&\quad\quad\quad\quad\small{\ce{H}}\\[-8pt] &\small{\diagdown\negthinspace\enspace\enspace\enspace\text{ }\enspace\space\text{ }\diagup}\\[-8pt] &\small{\quad\ce{C\bond{=}C}}\\[-8pt] &\small{\diagup\negthinspace\enspace\enspace\enspace\text{ }\enspace\space\text{ }\diagdown}\\[-8pt] \small{\ce{H}}&\quad\quad\quad\quad\small{\ce{H}}\\[4pt] \end{aligned} \quad \begin{aligned} \ce{+ }&\quad\ce{Br2}\quad \ce{->}\\[-5pt] \text{ }\\ \end{aligned} &\begin{aligned} &\small{\thinspace\quad\ce{Br}\negthinspace\quad\negthinspace\negthinspace\ce{Br}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|}\\[-5pt] &\small{\ce{H\bond{-}C\bond{-}C\bond{-}H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|}\\[-5pt] &\small{\negthinspace\quad\enspace\ce{H}\negthinspace\quad\negthinspace\negthinspace\thinspace\ce{H}}\\[4pt] \end{aligned}\\[-5pt] &\enspace\text{colourless } \quad\qquad\text{ orange}&\text{colourless} \end{aligned}\]Furthermore, alkenes can also be reacted with hydrogen in a process called hydrogenation. This process saturates the alkene, and the double bond opens up to the hydrogen, becoming an alkane. For example, the hydrogenation of ethene is denoted as:
\[\ce{C2H4 + H2 -> C2H6}\]A displayed formula can be used to better show the bond opening up to hydrogen and becoming saturated:
\[\begin{aligned} \small{\ce{H}}&\quad\quad\quad\quad\small{\ce{H}}\\[-8pt] &\small{\diagdown\negthinspace\enspace\enspace\enspace\text{ }\enspace\space\text{ }\diagup}\\[-8pt] &\small{\quad\ce{C\bond{=}C}}\\[-8pt] &\small{\diagup\negthinspace\enspace\enspace\enspace\text{ }\enspace\space\text{ }\diagdown}\\[-8pt] \small{\ce{H}}&\quad\quad\quad\quad\small{\ce{H}}\\[5pt] \end{aligned} \quad \begin{aligned} \ce{+ }&\quad\ce{H2}\quad \ce{->}\\[-5pt] \text{ }\\ \end{aligned} \enspace \begin{aligned} &\small{\negthinspace\quad\enspace\ce{H}\negthinspace\quad\negthinspace\negthinspace\thinspace\ce{H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|}\\[-5pt] &\small{\ce{H\bond{-}C\bond{-}C\bond{-}H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|}\\[-5pt] &\small{\negthinspace\quad\enspace\ce{H}\negthinspace\quad\negthinspace\negthinspace\thinspace\ce{H}}\\[5pt] \end{aligned}\]Alcohols
Alcohols are a homologous group similar to alkanes but instead of a hydrogen, there is the $\ce{O-H}$ functional group. Alcohols have a general formula of:
\[\ce{C}_n \ce{H}_{2n+1} \ce{OH}\]Where $n$ stands for the number of carbon atoms.
Using this formula, we can derive the formula of different alcohols:
\[\begin{aligned} &\small{\negthinspace\quad\enspace\ce{H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\text{ }\quad}\\[-5pt] &\small{\ce{H\bond{-}C\bond{-}O\bond{-}H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\text{ }\quad}\\[-5pt] &\small{\negthinspace\quad\enspace\ce{H}}\\[4pt] \text{Me}&\text{thanol}\ce{(CH3OH)} \end{aligned} \qquad\begin{aligned} &\small{\negthinspace\quad\enspace\ce{H\quad\negthinspace\negthinspace\negthinspace\thinspace H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|}\\[-5pt] &\small{\ce{H\bond{-}C\bond{-}C\bond{-}O\bond{-}H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|}\\[-5pt] &\small{\negthinspace\quad\enspace\ce{H\quad\negthinspace\negthinspace\negthinspace\thinspace H}}\\[4pt] \text{Et}&\text{hanol}\ce{(C2H5OH)} \end{aligned} \qquad\begin{aligned} &\small{\negthinspace\quad\enspace\ce{H\quad\negthinspace\negthinspace\negthinspace\thinspace H \negthinspace\quad\negthinspace\negthinspace\thinspace H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|\negthinspace\text{ }\quad|}\\[-5pt] &\small{\ce{H\bond{-}C\bond{-}C\bond{-}C\bond{-}O\bond{-}H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|\negthinspace\text{ }\quad|}\\[-5pt] &\small{\negthinspace\quad\enspace\ce{H\quad\negthinspace\negthinspace\negthinspace\thinspace H \negthinspace\quad\negthinspace\negthinspace\thinspace H}}\\[4pt] \text{P}&\text{ropanol}\ce{(C3H7OH)} \end{aligned} \qquad\begin{aligned} &\small{\negthinspace\quad\enspace\ce{H\negthinspace\negthinspace\quad\negthinspace\negthinspace\thinspace H\negthinspace\quad\negthinspace\negthinspace\thinspace H\negthinspace \quad\negthinspace\negthinspace\thinspace H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\negthinspace\quad|\text{ }\negthinspace\quad|\text{ }\negthinspace\quad|}\\[-5pt] &\small{\ce{H\bond{-}C\bond{-}C\bond{-}C\bond{-}C\bond{-}O\bond{-}H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\negthinspace\quad|\text{ }\negthinspace\quad|\text{ }\negthinspace\quad|}\\[-5pt] &\small{\negthinspace\quad\enspace\ce{H\negthinspace\negthinspace\quad\negthinspace\negthinspace\thinspace H\negthinspace\quad\negthinspace\negthinspace\thinspace H\negthinspace \quad\negthinspace\negthinspace\thinspace H}}\\[4pt] &\space\text{Butanol}\ce{(C4H9OH)} \end{aligned}\]When writing the chemical formula of alcohols, $\ce{OH}$ is always placed at the end to show the functional group. i.e. $\ce{CH3OH}$ instead of $\ce{CH4O}$. Furthermore, the $\ce{OH}$ group of alcohols can also be attached to different carbon atoms, or an alcohol may even contain multiple $\ce{OH}$ groups.
Carboxylic Acids
Carboxylic acids are a homologous group that have a functional group of $\ce{COOH}$. Carboxylic acids are all weak acids, which means they don’t fully ionise. When denoting carboxylic acids, the functional group $\ce{COOH}$ should always be at the end.
Carboxylic acids can be made by oxidising an alcohol with an oxidising agent. For example, potassium manganate(VII)($\ce{KMnO4}$) is an oxidising agent that can be used to oxidise ethanol.
\[\ce{CH3CH2OH ->[\ce{KMnO4}] CH3COOH}\\[5pt]\]This can be visualised using this displayed formula,
\[\begin{aligned} &\small{\negthinspace\quad\enspace\ce{H\quad\negthinspace\negthinspace\negthinspace\thinspace H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|}\\[-5pt] &\small{\ce{H\bond{-}C\bond{-}C\bond{-}O\bond{-}H}}\\[-5pt] &\small{\negthinspace\enspace\enspace\enspace\text{ }|\negthinspace\text{ }\quad|}\\[-5pt] &\small{\negthinspace\quad\enspace\ce{H\quad\negthinspace\negthinspace\negthinspace\thinspace H}}\\[4pt] \end{aligned} \quad \ce{->[\text{ }\ce{KMnO4}\text{ }]} \quad \begin{aligned} \small{\ce{H}}\enspace\quad&\enspace\enspace\small{\ce{O}}\\[-8pt] \small{|}\enspace\thinspace\quad&\small{\diagup \negthinspace\negthinspace\negthinspace\diagup}\\[-8pt] \small{\ce{H-C-C}} \\[-8pt] \small{|}\enspace\thinspace\quad&\small{\diagdown}\\[-8pt] \small{\ce{H}}\enspace\quad&\enspace\enspace\small{\ce{O-H}}\\[4pt] \end{aligned} \\[2pt] \small\ce{ethanol ->[\text{ potassium manganate(VII) }]} \small{\text{ ethanoic acid}}\]Carboxylic acids have a -oic acid ending and can be denoted with the general formula:
\[\ce{C}_{n-1}\ce{H}_{2n-1}\ce{COOH}\]Where $n$ stands for the number of carbon atoms.
Using this formula, we can derive the formula of different carboxylic acids:
\[\begin{aligned} &\enspace\enspace\small{\ce{O}}\\[-8pt] &\small{\diagup \negthinspace\negthinspace\negthinspace\diagup}\\[-8pt] \small{\ce{H-C}} \\[-8pt] &\small{\diagdown}\\[-8pt] &\enspace\enspace\small{\ce{O-H}}\\[4pt] \small{\text{Methanoic}}&\small{\text{ acid}\ce{(HCOOH)}} \end{aligned} \quad \begin{aligned} \small{\ce{H}}\thinspace\quad&\enspace\enspace\small{\ce{O}}\\[-8pt] \small{|}\thinspace\thinspace\quad&\small{\diagup \negthinspace\negthinspace\negthinspace\diagup}\\[-8pt] \small{\ce{H-C-C}} \\[-8pt] \small{|}\thinspace\thinspace\quad&\small{\diagdown}\\[-8pt] \small{\ce{H}}\thinspace\quad&\enspace\enspace\small{\ce{O-H}}\\[4pt] \small{\text{Ethonoic aci}}&\small{\text{d}}\small{\ce{(CH3COOH)}} \end{aligned} \quad \begin{aligned} \small{\ce{H}}\thinspace\thinspace\thinspace\thinspace\small{\ce{H}}\thinspace\quad&\enspace\enspace\small{\ce{O}}\\[-8pt] \small{|}\quad\thinspace\small{|}\thinspace\thinspace\quad&\small{\diagup \negthinspace\negthinspace\negthinspace\diagup}\\[-8pt] \small{\ce{H-C-C-C}} \\[-8pt] \small{|}\quad\thinspace\small{|}\thinspace\thinspace\quad&\small{\diagdown}\\[-8pt] \small{\ce{H}}\thinspace\thinspace\thinspace\thinspace\small{\ce{H}}\thinspace\quad&\enspace\enspace\small{\ce{O-H}}\\[4pt] \small{\text{Propanoic acid}}&\small{\ce{(C2H5COOH)}} \end{aligned} \quad \begin{aligned} \small{\ce{H}}\thinspace\thinspace\thinspace\thinspace\small{\ce{H}}\thinspace\thinspace\thinspace\thinspace\small{\ce{H}}\thinspace\quad&\enspace\enspace\small{\ce{O}}\\[-8pt] \small{|}\quad\thinspace\small{|}\quad\thinspace\small{|}\thinspace\thinspace\quad&\small{\diagup \negthinspace\negthinspace\negthinspace\diagup}\\[-8pt] \small{\ce{H-C-C-C-C}} \\[-8pt] \small{|}\quad\thinspace\small{|}\quad\thinspace\small{|}\thinspace\thinspace\quad&\small{\diagdown}\\[-8pt] \small{\ce{H}}\thinspace\thinspace\thinspace\thinspace\small{\ce{H}}\thinspace\thinspace\thinspace\thinspace\small{\ce{H}}\thinspace\quad&\enspace\enspace\small{\ce{O-H}}\\[4pt] \small{\text{Butanoic acid}}&\small{\ce{(C3H7COOH)}} \end{aligned}\]Polymerisation
Polymers are long chains of monomers.
Addition Polymerisation
Because alkenes are unsaturated, meaning that they have double bonds, sometimes the double bond can open up and allow the carbons to form new bonds with something else.
This means that multiple alkenes can open up their double bonds and join together to form polymer chains. This is called addition polymerisation.
For example, ethene($\ce{C2H4}$) can become polyethene($\ce{(C2H4)}_n$).
\[\begin{aligned} \Large n \quad\times \end{aligned} \begin{aligned} {\ce{H}}&\quad\quad\quad\quad{\ce{H}}\\[-8pt] &{\diagdown\negthinspace\enspace\enspace\enspace\text{ }\enspace\space\text{ }\diagup}\\[-8pt] &{\quad\ce{C\bond{=}C}}\\[-8pt] &{\diagup\negthinspace\enspace\enspace\enspace\text{ }\enspace\space\text{ }\diagdown}\\[-8pt] {\ce{H}}&\quad\quad\quad\quad{\ce{H}}\\[4pt] \end{aligned} \begin{aligned} \quad\ce{->[ \text{ catalyst } ][ \text{ pressure }]}\quad \end{aligned} \begin{aligned} \Bigg( \kern-1em \begin{aligned} &\enspace\thinspace\thinspace\ce{H}\enspace\thinspace\ce{H}\\[-5pt] &\quad|\quad\thinspace|\\[-5pt] &\ce{\bond{-}C\bond{-}C\bond{-}}\\[-5pt] &\quad|\quad\thinspace| \\[-5pt] &\enspace\thinspace\thinspace\ce{H}\enspace\thinspace\ce{H} \end{aligned} \kern-1em\Bigg)_{\Large{n}} \end{aligned}\\ \text{many single ethenes } \ce{->[\text{ catalyst }][\text{ pressure }]} \text{ polyethene}\]To write the formula of the polymer, simply put the formula of the monomer in brackets followed by a subscript $n$.
Condensation Polymerisation
Condensation polymerisation typically involves two different types of monomers. Each monomer contains at least two functional groups, which partially break off and react to form a small molecule like water.
✏✏✏
Polyesters form when carboxylic acid monomers and alcohol monomers react together
\[\ce{C\bond{-}}\blacksquare\negthinspace\blacksquare\negthinspace\blacksquare\ce{\bond{-}C}\]Polyamides are formed from carboxylic acid monomers and amine monomers.
\[idk\]Naturally Occurring Polymers
There are many important naturally occurring polymers, for example, DNA is a complex molecule that contains genetic information. It contains two strands of nucleotide monomers that bond together in a polymerisation reaction. There are four types of nucleotide monomers in DNA,
\[\begin{aligned} \text{Adenosine monophosphate(A) - }&\ce{C10H14N5O7P}\\ \text{Guanosine monophosphate(G) - }&\ce{C10H14N5O8P}\\ \text{Cytidine monophosphate(C) - }&\ce{C9H14N3O8P}\\ \text{Thymidine monophosphate(T) - }&\ce{C10H14N2O8P^{1−}}\\ \end{aligned}\]Furthermore, amino acids form protein polymers via condensation polymerisation. Proteins are incredibly important for the function of the human body.
Other polymers like starch and cellulose are large and complex carbohydrates, which are molecules that contain carbon, oxygen and hydrogen.
Fuel
Crude Oil
Crude oil is a fossil fuel and is non-renewable as it is made much slower than it is being extracted. This is why it is called finite resource.
Crude oil is a mixture of different hydrocarbons, so after drilling up crude oil, the different compounds in crude oil are separated using fractional distillation. This works as longer hydrocarbons have higher boiling points and vice versa.
This is because longer hydrocarbons have more intermolecular forces than shorter hydrocarbons, and so are harder to turn into gas.
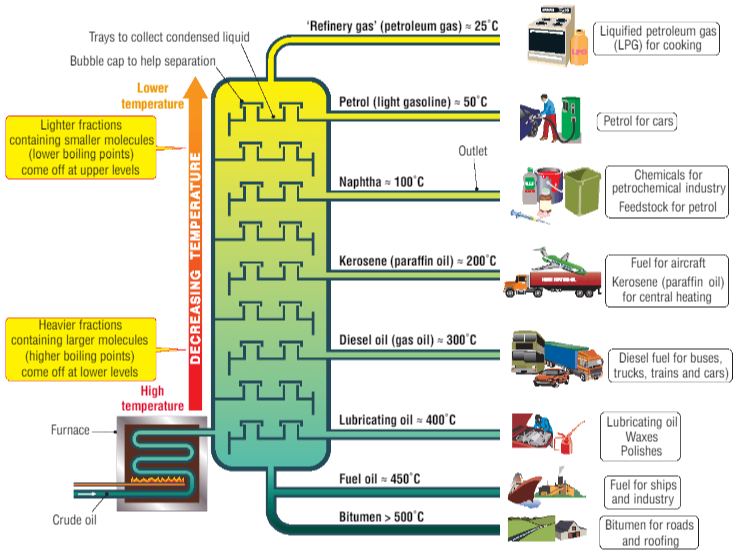
Fractional distillation of crude oil. From: Quizlet
Cracking
Cracking is a thermal decomposition reaction, which breaks down longer alkane molecules into smaller alkane and alkene molecules. Because this means breaking bonds, cracking requires large amounts of heat, and generally also a catalyst to increase the rate of reaction.
-
Vaporised hydrocarbons are passed over powdered catalyst at around $400-700°\mathrm{C} $ and $70 \mathrm{atm}$.
-
Aluminium oxide is used as a catalyst. This causes the long-chain molecules to split apart of crack on the surface of the bits of catalyst.
Cracking helps meet supply and demand as it introduces a way for less used longer alkanes to be cracked into shorter, more useful alkanes and alkenes.
Fuel Cells
Fuel cells can be used to generate electricity using hydrogen and oxygen.
Chemical cells include the batteries
Global warming and pollution
The Atmosphere
The early atmosphere was composed of mostly carbon dioxide, however, after algae started forming and photosynthesised, the carbon dioxide in the atmosphere started to release oxygen
The current composition of the atmosphere is around $78\%$ nitrogen, $21\%$ oxygen, $0.04\%$ argon and $0.04\%$ carbon dioxide.
The Greenhouse Effect
Global warming
Pollution
Pollutants are substances released into the atmosphere that may harm living things.