Physics revision notes more details will be placed here
P1 - Matter
The Atom
The History of the Atomic Model
In 1897, JJ Thomson conducted several experiments which led him to the conclusion that atoms weren’t just solid spheres like had been thought before. His model proposed an atom that consisted of negative particles(electrons) spread throughout a solid sphere. This was known as the plum pudding model.
In 1909, Ernest Rutherford with the help of Hans Geiger and Ernest Marsden, conducted the gold foil experiment. In which a beam of positively charged Alpha Particles was shot at a gold foil surrounded by a screen.
It was expected for the majority of the particles to pass straight through the foil, unaffected. However, during the experiment, a large number of particles diverged with some even deflecting of off the gold foil.
This led to Rutherford proposing the nuclear model in 1911. This model presented the atom as having the majority of its mass concentrated in a tiny, positive nucleus. Surrounded by a cloud of negative electrons.
However, scientists soon realised that this model had its flaws. Due to the negative nature of electrons and the positive nature of the nucleus. An atom following Rutherford’s model would collapse instantly due to electrostatic forces of attraction.
Finally, a few years later in 1913, Niels Bohr proposed a new model where electrons only existed in shells or fixed orbits based on its energy level.
Properties of the atom
The nucleus contains protons and neutrons and is extremely small. With a radius of about $1\times 10^{-15}\textrm{m}$. Almost all the mass of atom (about $1\times10^{-23}\textrm{g}$), is concentrated in the nucleus.
The rest of an atom is mostly empty space. The negative electrons orbit around the nucleus and give it its overall size - a diameter of around $1\times10^{-10}\textrm{m}$. This means that the radius of the nucleus is around 10,000 times smaller than the radius of the atom.
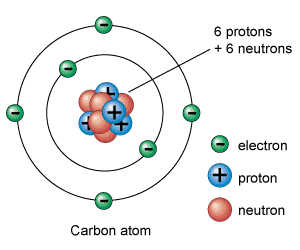
Atom Model. By: Universe Today
| Particle | Relative mass | Relative charge |
|---|---|---|
| Proton | 1 | +1 |
| Neutron | 1 | 0 |
| Electron | 0.0005 | -1 |
Density and particles
Density
Density is the amount of how much mass a substance has, per unit volume. To find density, you can just divide a substance’s mass by its volume.
\[\rho ={\frac {m}{V}}\]$\rho$ = density
$m$ = mass
$V$ = volume
The units of density can be $\textrm{g}/\textrm{cm}^3$ or $\textrm{kg}/\textrm{m}^3$. The average density of an object will determine whether it it floats or sinks. For example, wood has a lower average density than water, therefore it floats.
Particle Theory
Particle theory is used to help us better understand states of matter, it considers each of the particles in a certain state as small, solid and inelastic sphere.
Solids
Solids have strong forces of attraction between particles, this holds them together in a fixed, regular lattice structure. Because the particles are fixed, the overall substance keeps a definite shape and volume.
Liquids
Liquids have weak forces of attraction between particles, this means they are free to move around and form irregular arrangements. However, due to there being forces of attraction, particles tend to stay together and the substance tends to keep its overall volume, even if the shape changes.
Gas
Gases have very weak forces of attraction, which means they are free to move, this means that they do not keep a definite shape or volume. But rather moves to fill any volume of space.
Change of state
graph RL;
solid-- melting -->liquid;
liquid-- freezing -->solid;
liquid-- evaporating -->gas;
gas-- condensing -->liquid;
gas--deposition-->solid;
solid--sublimation-->gas;
When substances are heated or cooled, energy is taken or added to the particles kinetic energy stores. Once particles gain/lose enough kinetic energy to change state, bonds are broken/made depending on which direction the state is changing.
In addition, the mass never changes when a substance changes state. However, volume and density will change. Generally substances are more dense when they are solid and less dense as gases.
Heat capacity and Latent heat
Specific Heat Capacity
Specific heat capacity is the amount of energy released/needed to raise/lower the temperature of $1\rm{kg}$ of a substance by $\rm{1\rm{°C}}$.
What is temperature?
When a substance is heated, the particles in that substance gain kinetic energy and move faster. Temperature is actually the measure of the average internal kinetic energy of a substance.
Different substances need different amounts of energy to heat up.
For example, to warm up $1\rm{kg}$ of water by $\rm{1\rm{°C}}$, $4200\rm{J}$ are required. However, to heat up $1\rm{kg}$ of mercury by $\rm{1\rm{°C}}$, only $139\rm{J}$ are required.
Therefore, the specific heat capacities of those substances is that amount of energy, and is measured in $\rm{J}/\rm{kg}\rm{°C}$ . Specific heat capacity can also be defined as the amount of energy released when a substance cools, both yield the same results.
\[Q = mc\Delta T\]$Q$ = heat energy
$m$ = mass
$c$ = specific heat capacity
$\Delta T$ = change in temperature
Finding specific heat capacity
✏✏
Specific Latent Heat
During state changes, energy that is put into the system is not used for increasing temperature, but is used for breaking/making bonds that complete the change in state. This can be seen from the graph of gradually raising a substance’s temperature.
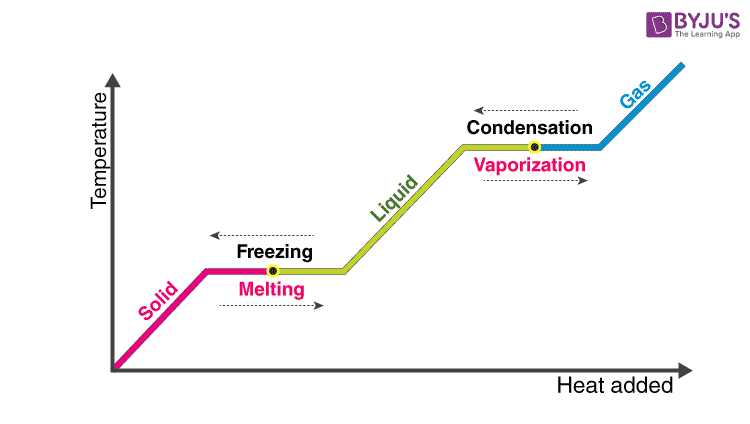
Line Graph of Latent Heat By: BYJU
The amount of energy it takes to change state is called the latent heat, however, this varies depending on the mass and type of substance.
To standardize these values, specific latent heat is used, this is the amount of energy needed/released in a change of state of $1\rm{kg}$ of a substance. Specific latent heat is measured using the unit: $\rm{J}/\rm{kg}$
\[Q = m \times l\]$Q$ = heat energy
$m$ = mass
$l$ = specific heat capacity
Pressure
Gas Pressure
In a gas, particles are constantly moving around, colliding with each other, and the container. These collisions cause gas pressure, as they exert force on the container.
The more particles there are in a given volume, the higher the gas pressure.
The higher the temperature, the higher the pressure, as particles move around faster as they have more kinetic energy and therefore exert more force on the container.
Gas pressure can be explored using this simulator.
When there is a fixed amount of gas at a constant temperature, whenever volume increases, pressure will decrease, and whenever pressure increases, volume has to decrease.
This is called Boyle’s Law and results in the equation:
\[pV = k \text{ }\text{ } \text{ or } \text{ } \text{ } p\propto \frac{1}{V}\]$p$ = pressure
$V$ = volume
$k$ = constant
This equation shows that, as volume increases, the pressure of the gas decreases in proportion. Similarly, as volume decreases, the pressure of the gas increases.
However, when the container of the gas is flexible, such as a balloon, a rise in gas temperature will cause a force to be exerted on the inside walls of the container, causing it to expand until it has matched the outside pressure, or the container breaks.
Atmospheric Pressure
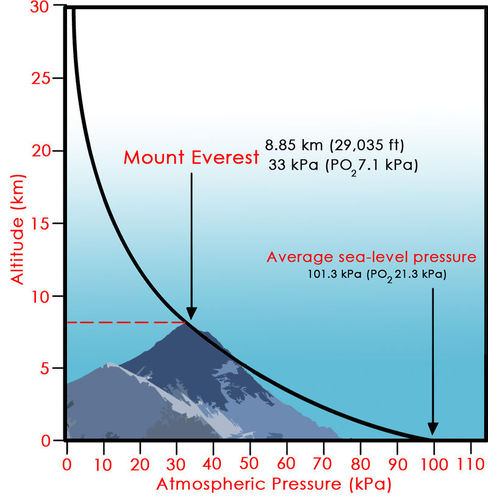
Line Graph of altitude to atmospheric pressure. By: FlexBooks
The lower you are, the more air particles exert force on you from above, and the higher the density of air particles around you. This causes higher atmospheric pressure the closer you are to the ground.
This is why hikers have to carry oxygen tanks with them when traversing high altitude landscapes, as the air is much less dense and there are fewer oxygen particles that are necessary for human function.
Liquid Pressure
When an object is submerged or partially submerged in a liquid, it experiences liquid pressure from all directions due to the liquid particles. This pressure will increase with depth due to the weight of the liquid above the object.
The pressure at any given depth is given by the equation:
\[p = \rho g h\]$p$ = pressure due to column of liquid
$\rho$ = density of liquid
$g$ = gravitational acceleration
$h$ = height of column
P2 - Forces
Motion
Scalers and Vectors
Scalers are quantities that just have a magnitude(value/size). Scaler quantities include things such as: mass, distance, speed and temperature.
Vectors are quantities that have magnitude and direction, such as velocity, displacement, force, and acceleration.
For example, a car moving in one direction could have the** velocity** of $3 \text{ m/s}$, but moving the opposite direction would have a a velocity of $-3 \text{ m/s}$. However, in both cases, the car has a speed of $3 \text{ m/s}$ .
Speed and Velocity
Speed is a scaler, and is the measure of the amount of distance travelled per unit time, the equation for speed is denoted as:
\[s = \frac{d}{t}\]$s$ = speed
$d$ = distance travelled
$t$ = time elapsed
The equation for velocity is similar, however, the velocity of an object will most likely not be constant, and so this equation is used to calculate the average velocity of an object over a period of time:
\[\bar{v}=\frac {\Delta x}{\Delta t}\]$\bar{v}$ = average velocity
$\Delta x$ = displacement
$\Delta t$ = elapsed time
Acceleration
Acceleration is the rate of change of velocity and is measured in $\text{m/s}^2$ as it is the amount that velocity has changed per unit time.
To calculate and describe motion in a given direction when acceleration is constant, $\text{SUVAT}$ equations can be used.
$\boldsymbol{s}$ = displacement
$\boldsymbol{u}$ = initial velocity
$\boldsymbol{v}$ = final velocity
$\boldsymbol{a}$ = acceleration
$t$ = time
The first $\text{SUVAT}$ equation can be used to find any of the unknowns.
\[\begin{equation} v = u + at \end{equation} \tag{1}\]It can be rearranged into $a = \frac{v-u}{t}$ which can be used to find acceleration as acceleration equals change in velocity($v-u$) divided by time($t$).
This equation can be used to find any unknowns when displacement is involved.
\[\begin{equation} v^2 = u^2 + 2as \end{equation} \tag{2}\]These 2 equations can be used to find any unknowns when an initial or final velocity is not known.
\[\begin{equation} s = ut + \frac{1}{2}at^2 \end{equation} \tag{3}\] \[\begin{equation} s = vt - \frac{1}{2}at^2 \end{equation} \tag{4}\]Finally, this equation can be used when acceleration is unknown.
\[\begin{equation} s = \frac{1}{2}(u+v)t \end{equation} \tag{5}\]Investigating motion
✏✏✏✏
Motion Graphs
Distance-time graphs
Distance-time graphs plot distance to time, thus, the gradient of the graph represents the speed of the object at that time. This is because the gradient of a graph is $\frac{\text{change in }y}{\text{change in }x}$ and on a distance time graph this would be $\frac{\text{distance}}{\text{time}}$, which is the equation of acceleration.
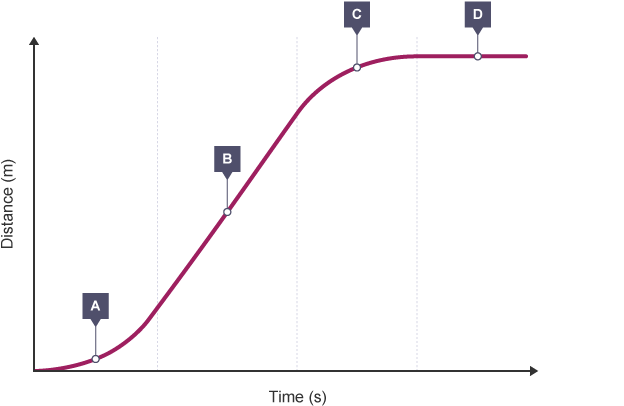
Distance Time Graph: BBC Bitesize
| Section of Graph | Gradient | Speed | Acceleration |
|---|---|---|---|
| $A$ | increasing | increasing | accelerating |
| $B$ | constant | constant | not accelerating |
| $C$ | decreasing | decreasing | decelerating |
| $D$ | none | stationary | stationary |
If the graph is curved, that means that the object is either decelerating or accelerating. To find the exact speed of the object at a certain point in time during during acceleration or deceleration, a tangent can be drawn and the gradient of the tangent can be found.
Velocity-time graphs
Velocity time graphs plot velocity to time, this allows us to find acceleration using the gradient as acceleration is equal to $\frac{\text{velocity}}{\text{time}}$.
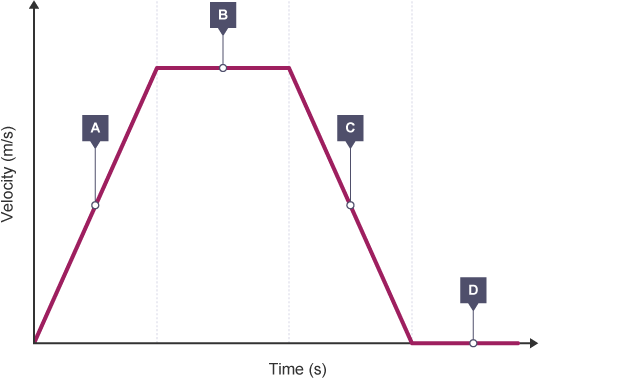
Velocity Time Graph: BBC Bitesize
| Section of Graph | Gradient | Acceleration | Speed/Velocity |
|---|---|---|---|
| $A$ | positive | accelerating | increasing |
| $B$ | none | none | constant |
| $C$ | negative | decelerating | decreasing |
| $D$ | zero | none | stationary |
Finally, the area under the graph up till a certain time will give you the amount of distance travelled within that time.
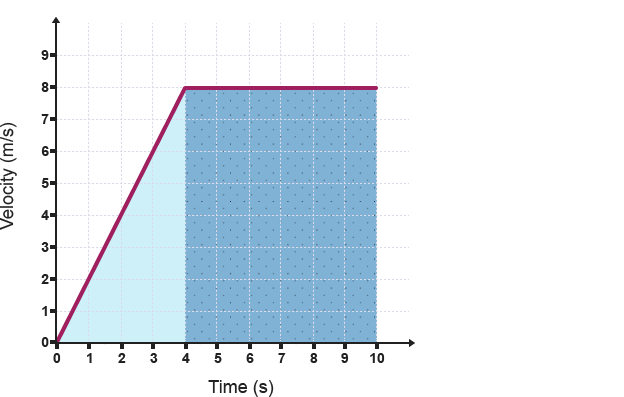 Velocity Time Graph: BBC Bitesize
Velocity Time Graph: BBC Bitesize
For example, to find the amount of distance travelled by the object above in $4$ minutes, the area of the light blue triangle can be found, $\frac{8\times4}{2} = 16 \mathrm{m}^2$. Therefore the total distance travelled by the object in $4$ minutes is $16\mathrm{m}$.
To find the distance travelled for the whole $10$ minutes, we can find the area of the dark blue rectangle and add the area’s together to find the entire area under the graph. $16 +(6\times8) = 64\mathrm{m}$ travelled.
Force diagrams
Contact and Non-Contact Forces
To exert a contact force, two objects must be in contact, for example, friction, tension and air resistance are all examples of contact forces.
Non-contact forces, on the other hand, do not require two objects to be touching. These include the electrostatic, magnetic and gravitational forces.
Finally, the normal contact force is a contact force that is a type of reaction force, and acts at right angles to the surface. This force is a representation of Newton’s third law: “When two objects interact, the forces they exert on each other are equal and opposite”.
Free Body diagrams and Resultant force
In most situations, an object has multiple forces acting on it, for example, weight and the normal contact force. Because forces are vectors, they have magnitude and direction, this can be visualised using arrows in a free body diagram.
Longer arrows represent greater force, and the direction of the force is represented by the direction of the arrow.
\[\begin{aligned} &\thinspace\thinspace\Big \uparrow 6\mathrm{N}\\ &\LARGE{\blacksquare} \\[-5pt] &\thinspace\thinspace\bigg \downarrow 15\mathrm{N} \end{aligned} \qquad \begin{aligned} \text{resultant force:}\\ \lvert 15\mathrm{N} - 6\mathrm{N}\rvert = 9\mathrm{N} \downarrow\\ \end{aligned}\]Furthermore, when there are multiple forces acting on an object, the difference between the forces can be found, this is the resultant force. Which is the overall force acting on an object.
If the resultant force of an object is $0$, then it will either be stationary or moving at a constant speed as no forces are acting on it. This is called equilibrium.
However, if the resultant force is greater or less than $0$, then the object is accelerating or decelerating.
\[\begin{aligned} &\thinspace\thinspace\Big \uparrow \small\text{normal contact force: }10 000\mathrm{N}\\[-5pt] \small\text{thrust: }1900\mathrm{N}\normalsize\longleftarrow &\negthinspace\negthinspace\negthinspace\LARGE{🚓}\negthinspace\negthinspace\negthinspace\negthinspace\negthinspace\normalsize\rightarrow\small\text{drag: }900\mathrm{N}\\[-5pt] &\thinspace\thinspace\Big \downarrow\small\text{weight: } 10 000\mathrm{N} \end{aligned} \quad \begin{aligned} \text{resultant force:}\\ \lvert 10000\mathrm{N} - 10000\mathrm{N}\rvert = 0\mathrm{N} \updownarrow\\ \lvert 1900\mathrm{N} - 900\mathrm{N}\rvert = 1000\mathrm{N} \leftarrow \end{aligned}\]Finding multi-dimensional resultant forces
To find the resultant force with two forces at right angles, we can use Pythagoras theorem to find the resultant force.
\[a^2 + b^2 = c^2\]$a$ = side of right angled triangle
$b$ = side of right angled triangle
$c$ = hypotenuse
Thus, if we treat the following free-body diagram as a triangle with sides $15$ and $8$, we can find the resultant force by finding the hypotenuse.
\[\begin{aligned} &\thinspace\thinspace\bigg \uparrow 15\mathrm{N}\\ &\LARGE{\blacksquare} \normalsize \longrightarrow 8\mathrm{N}\\[-5pt] \end{aligned} \qquad \begin{aligned} \text{resultant force:}\\ \sqrt{15^2+8^2}=\\ \sqrt{289}= 17\mathrm{N} \nearrow\\ \end{aligned}\]Newton’s laws
Newton’s First Law of Motion
Newton’s first law states that
“An object at rest stays at rest and an object in motion stays in motion with the same speed and in the same direction unless acted upon by an unbalanced force.”
This means that if any number of external forces $\displaystyle{\mathbf{F_1}, \mathbf{F_2}, \ldots }$ are being applied to an object, then the resultant force $F_{\text{Net}}$ would be the vector sum of those forces.
If the resultant force is $0$, then the object’s velocity must not be changing. This also means that if the object’s velocity is not changing, the resultant force or net force is $0$.
This can be notated as:
\[\mathbf{F} _{\text{Net}}=0\;\Leftrightarrow \;{\frac {\Delta v }{\Delta t}}=0 \tag{1}\]$\mathbf {F} _{\text{Net}}$ = resultant/net force
$\Delta v$ = change in velocity
$\Delta t$ = time elapsed
If there is a non-zero resultant force, then the object will accelerate or decelerate in the direction of said force.
Newton’s Second Law of Motion
Newton’s second law states that
“The rate of change of momentum of a moving body is proportional to the force acting to produce the change.”
This law relates acceleration and force and states that they are proportional by the object’s mass. Newton’s second law can be denoted as:
\[\mathbf{F} _{\text{Net}} =m a \tag{2}\]$\mathbf {F} _{\text{Net}}$ = resultant/net force
$m$ = mass
$a$ = acceleration
Intertia
Newton’s second law also explains inertia. The more massive an object, the more force it requires to alter or change its acceleration. This is because $\text{mass}=\frac{\text{force}}{\text{acceleration}}$.
Newton’s Third Law of Motion
Newton’s third law states that
“To every action there is an equal and opposite reaction.”
This means that when two objects interact, they exert the same force on each other.
So, if one object $A$ exerts force $\boldsymbol{\mathrm{\overrightarrow{F}}}_A$ on a second object $B$, then $B$ simultaneously exerts a force $\boldsymbol{\mathrm{\overrightarrow{F}}}_B$ on $A$. These two forces have the same magnitude but an opposite direction and so can be shown as:
\[\boldsymbol{\mathrm{\overrightarrow{F}}}_A = -\boldsymbol{\mathrm{\overrightarrow{F}}}_B \tag{3}\]
Newton’s third law. From: Wikimedia Commons
Friction, momentum and weight
Terminal Velocity
When an object is falling, it will accelerate due to gravitational forces, however, as velocity increases, so does air resistance. This is because resistance is directly proportional $\text{resistance} \propto \text{velocity}$.
This means that the greater the velocity becomes due to acceleration, the more air resistance the object will feel. This happens until a point where the velocity and air resistance are the same. So that the resultant force is $0$. Meaning that there is no more acceleration and the object falls at a constant velocity.
Momentum
Momentum is a property of moving objects that relates its mass and its velocity. The higher the mass, the higher the momentum. The higher the velocity, the higher the momentum.
\[p = m v\]$p$ = momentum
$m$ = mass
$v$ = velocity
Momentum is a vector, meaning that it can be positive or negative and has direction.
Relating force to momentum
We can derive how force relates to momentum using Newton’s second law.
Newton’s second law is $F =m a$, acceleration is change in velocity over time elapsed $a=\frac{\Delta v}{\Delta t}$ . So Newton’s second law can also be written as $F = \frac{m\times\Delta v}{\Delta t}$. Mass multiplied by change in velocity is the same as change in momentum and so we can write the equation relating force to momentum as:
\[F = \frac{\Delta p}{\Delta t}\]$F$ = force
$\Delta p$ = change in momentum
$\Delta t$ = elapsed time
This means that:
-
The faster a change in momentum happens(the smaller $\Delta t$ is), the greater the force($F$).
-
The larger the change in momentum(the bigger $\Delta p$ is), the greater the force.
Conservation of Momentum
In a closed system, the total momentum remains constant. This is the law of conservation of momentum. If two objects interact, the final momentum is conserved.
This can be deduced from the equation relating force to momentum.
If object one and object two are subject to a force before the collision $F_1 = \frac{\Delta p_1}{\Delta t}$ and $F_2 = \frac{\Delta p_2}{\Delta t}$, then, according to Newton’s third law, during the collision:
\[F_1 = -F_2\Leftrightarrow\frac{\Delta p_1}{\Delta t} = -\frac{\Delta p_2}{\Delta t}\]Because the momentum is equal to velocity multiplied by mass, we can define the velocities of the objects before the collision as $u_1$ and $u_2$, and afterwards as $v_1$ and $v_2$. This means that,
\[\frac{m_1(v_1-u_1)}{\Delta t} = -\frac{m_2(v_2-u_2)}{\Delta t}\]Therefore,
\[\begin{aligned} m_1v_1-m_1u_1 &= -m_2v_2+m_2u_2\\ m_1u_1 + m_2 u_2 &= m_1 v_1 + m_2 v_2 \end{aligned}\]This means that the total momentum of both objects before the collision $\Sigma p$ is equal to the total momentum of both objects after the collision $\Sigma p’$.
\[\therefore \Sigma p = \Sigma p'\]Weight
Weight is a the force due to gravity that affects objects with mass. The stronger the gravitational field strength, the higher the weight, though the mass stays constant.
The earth has a gravitational field strength of around $9.8 \mathrm{N/kg}$ or $9.8 \mathrm{m/s}^2$. To find the amount of weight an object has, the following equation can be used.
\[F = mg\]$F$ = weight
$m$ = mass
$g$ = gravitational field strength
Energy and Force
Mechanical Energy Stores
Gravitational Potential Energy Stores
When an object is at any height above the earth’s surface, it has gravitational potential energy. To calculate this energy, the following equation can be used.
\[E_{p} = mgh\]$E_p$ = gravitational potential energy
$m$ = mass
$g$ = gravitational field strength
$h$ = height
When an object falls, it loses energy from its gravitational potential energy store, and when it reaches the ground, its gravitational potential energy is $0 \mathrm{J}$.
Kinetic Energy Stores
When an object moves, it has energy in its kinetic energy store. This energy depends on the object’s mass and velocity.
\[E_k = \frac{1}{2}mv^2\]$E_k$ = kinetic energy
$m$ = mass
$v$ = velocity
This means that doubling the mass of an object will double its kinetic energy. If you double the velocity, the energy in the kinetic energy store will quadruple.
Work Done and Power
Work Done
Work done is the amount of energy transferred in this case by a mechanic force. To find the amount of work done, the following equation can be used.
\[W = Fs\]$W$ = work done
$F$ = force
$s$ = displacement along line of action
Since work done is the amount of energy transferred, it is also equal to the change in energy transferred.
\[W = \Delta E_k\]$W$ = work done
$\Delta E_k$ = change in energy transferred
Power
Power is the rate at which energy is transferred. It is measured in watts($\mathrm{W}$) which is equivalent to the amount of joules($\mathrm{J}$) transferred per second($\mathrm{s}$) which means $1\mathrm{W} = 1\mathrm{J/s}$.
\[P = \frac{W}{\Delta t}\]$P$ = power
$W$ = work done
$\Delta t$ = elapsed time
Elasticity and Moments
Elasticity
When you apply a force to an object it can be stretched, compressed or bent. This is called deformation, in order to deform an object, at least two forces are needed.
Plastic deformation is when the object does not return to its original shape after being defmored.
Elastic deformation is when the object returns to its original shape after being deformed.
Hooke’s Law
When extending a spring, the extension of the spring is directly proportional to the amount of force exerted on the spring. This relationship is called Hooke’s law and uses the spring constant($\mathrm{N/m}$) which is a measure of the amount of force($\mathrm{N}$) it takes to extend the spring by $1 \mathrm{m}$.
\[F_s = kx\]$F_s$ = force exerted
$k$ = spring constant
$x$ = spring extension
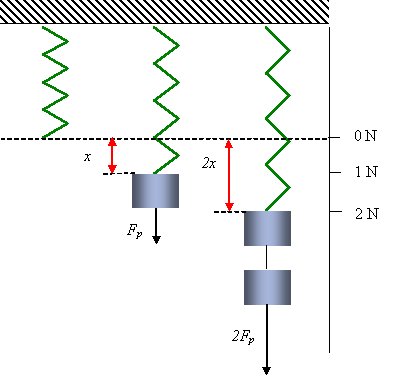
Hooke’s Law. From: Boundless Physics
The stiffer the spring, the larger the spring constant, as it takes more force to stretch the spring by $1 \mathrm{m}$.
Elastic limit
However, once a spring reaches a certain limit known as the elastic limit, it will stop being stretched elastically and instead become plastic deformation, which will not return to its original form.
Work done to deform
When a force deforms an object, energy is transferred and thus work is done. To find out the amount of energy transferred during elastic deformation, the following equation can be used.
\[W = \frac{1}{2}kx^2\]$W$ = work done
$k$ = spring constant
$x$ = spring extension
You can also find the work done by finding the area under a force-extension graph.
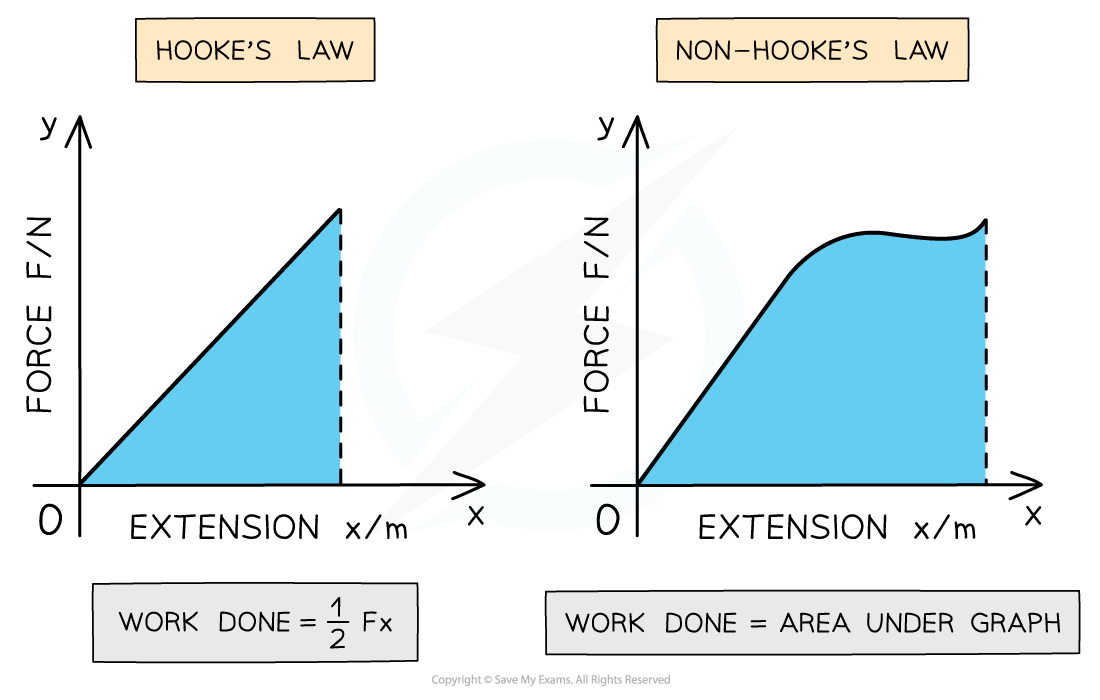
Work done on spring. From: Save My Exams
Moments
If a force acts on an object with a pivot, like a door or a hinge, it can cause the object to rotate around the pivot. The moment of a force is the turning effect of it, or its tendency to cause a body to rotate around an axis. The moment of a force is measured in newton-meters($\mathrm{Nm}$) as it is the relationship between the distance($\mathrm{m}$) from the pivot and the force($\mathrm{N}$) acting on it.
The size of a moment can be given by,
\[\mu_{n} = r^{n}Q\]$\mu_{n}$ =moment
$r^{n}$ = distance from pivot
$Q$ = physical quantity/ force
Levers and Gears
Levers
Levers are known as force multipliers as they reduce the force need to get the same moment.
Because the moment of the input force is the same as the moment of the output force, and because $\mu_{n} = r^{n}F$, we can write a rule relating input force and output forces.
\[\frac{F_1}{F_2} = \frac{r^{n}_1}{r^{n}_2}\]$F_1$ = input force
$F_2$ = output force
$r^{n}_1$ = distance of output force from pivot
$r^{n}_2$ = distance of input force from pivot
Gears
✏✏✏
Hydraulics
Pressure in a fluid(liquid or gas) is caused by the kinetic energy of particles in the fluid. This causes pressure to be transmitted equally in all directions and it causes a force at right-angles to any surface.
Pressure is the amount of force per meter squared, which is measured in pascals($\mathrm{Pa}$). The equation for pressure is,
\[P = \frac{F}{A}\]$P$ = pressure
$F$ = force
$A$ = area
Because liquids such as water are incompressible, hydraulic systems can be used as force multipliers. This is because pressure is maintained the same throughout the system, so a larger cross-sectional area will increase the output force.
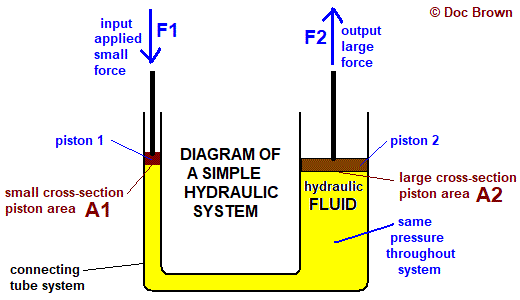
Hydraulic system. From: Doc Brown’s Chemistry
P5 - Waves
What are Waves?
Waves transfer energy from one location to another without transferring matter. Waves can have amplitude, wavelength and frequency.
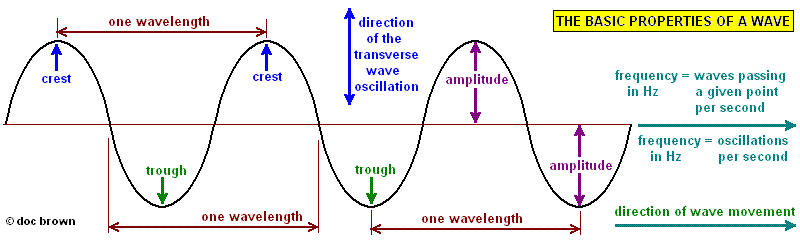
Wave properties. From: Doc Brown’s Chemistry
The period of a wave is the amount of time it takes for one full cycle or $T = \frac{1}{f}$ where $T$ is period and $f$ is frequency.
The speed of a wave or the wave speed can be calculated with $v = f\lambda$, where $v$ is wave speed, $f$ is frequency and $\lambda$ is wavelength.
There are two main types of waves, longitudinal waves and transverse waves.
Transverse Waves
In transverse waves, the vibrations are perpendicular to the direction the wave travels. Tranverse waves can travel on the surface of liquids, however, they cannot travel through liquids. Examples of transverse waves include electromagnetic radiation, S seismic waves and ripples on the surface of water.
Longitudinal Waves
In longitudinal waves, the vibrations are parallel to the direction the wave travels. Longitudinal waves have compressions(areas of high pressure) as peaks and rarefactions(areas of low pressure) as troughs.
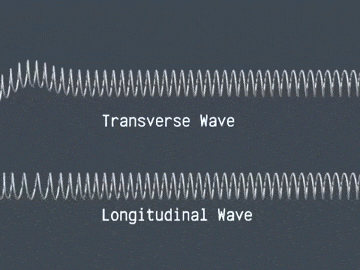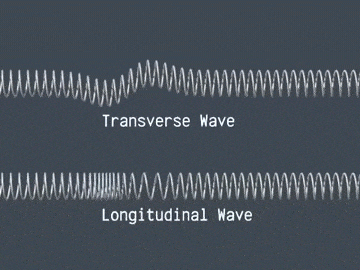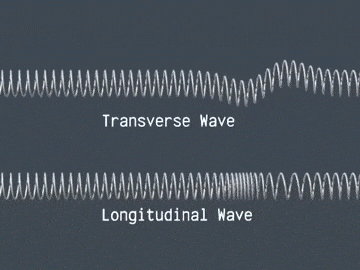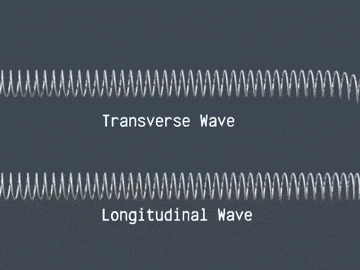
Waves. From: Study Rocket
Examples of longitudinal waves include soundwaves and P seismic waves.
Ripple tank
✏✏✏
Reflection and Refraction
At a boundary, waves can do one of four things: absorbed, transmitted, reflected or boundaries.
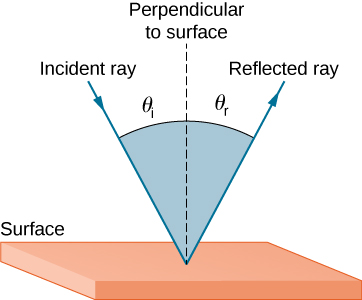
Reflection
When light is reflected on a material, its angle of incidence will equal its angle of reflection.
\[\theta_r = \theta_i\]$\theta_r$ = angle of reflection
$\theta_i$ = angle of incidence
The angle of incidence and angle of reflection are perpendicular to the surface.
The law of Reflection. From: OpenStax
However if the surface is rough, like paper, the light will not be reflected evenly, and so it will appear diffused.
Refraction
Refraction occurs when a wave is transmitted at a boundary. Refraction causes a change in speed for the wave due to different densities.
Electromagnetic waves
EM waves travel slower in denser materials, however, they keep their frequency. However, because $v=f\lambda$, if speed decreases and frequency stays the same, then wavelength must decrease. Refraction of light follows Snell’s law, which states that, the ratio of the sines of the angle of incidence and angle of reflection is equal to the ratio of the velocity or indices of refraction(how fast light travels through the material).
\[\frac{\sin\theta_1}{\sin\theta_2}=\frac{v_1}{v_2}=\frac{n_1}{n_2}\]$\theta_1$ = angle of incidence
$\theta_2$ = angle of refraction
$v_1$ = phase velocity of medium 1
$v_2$ = phase velocity of medium 2
$n_1$ = refractive index of medium 1
$n_2$ = refractive index of medium 1
However, when the speed of a wave changes, its direction changes relative to the $90°$ normal. The slower the wave, the greater the change in direction.
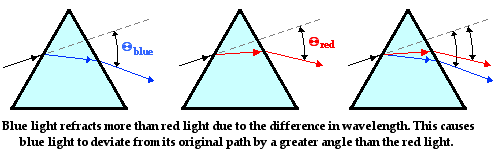
Dispersion of Light by Prisms. From: The Physics Classroom
As blue light has a lower wavelength and higher frequency, it is slowed down more when it enters a different density.
Sound waves
Hearing
Sound waves generally travel faster in higher density materials, this is because the particles are closer together, making it easier for particles to vibrate each other to pass the sound wave.
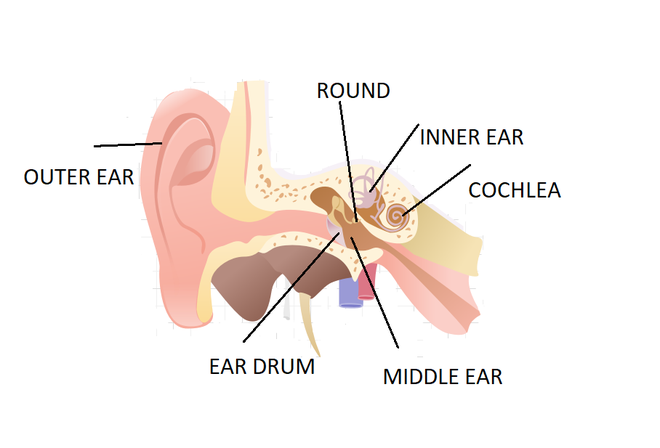
How do we hear? From: GeeksforGeeks
When sound waves reach the outer ear, it travels into the ear and causes the ear drum to vibrate. These vibrations pass to tiny bones in the ear called ossicles, through the ear canal to the cochlea. The cochlea contains fluid that moves tiny hairs which send electrical signals to your brain.
Young people can hear frequencies ranging $20 \mathrm{Hz}$ to $20000 \mathrm{Hz}$, as people age, the upper limit of their hearing decreases, this is mainly due to wear and tear of the cochlea and auditory nerve.
Sonar and Ultrasound
When a wave reaches a boundary, it can be partially reflected based on the medium. This means that if we know the speed of the wave we can use $d = vt$ to find the distance away the medium is based using a detector.
This technology is used in the medical industry, where ultrasound waves are passed through the body to visualise a baby in a pregnant woman’s stomach. This works by calculating the distance between different boundaries, which can be processed by a computer.
Another use for ultrasound is in construction, where ultrasound can be used to detect pipes or other materials behind walls.
Sonar
Sonar is a type of sound wave used by boats and submarines to find the distance to the seabed or to locate objects under the water. This can be done using a transmitter and a receiver.
Electromagnetic waves
Electromagnetic waves are transverse waves that travel at a constant speed through a vacuum. This speed is defined as $c$ and which is equal to exactly $299792458\text{ }\mathrm{m/s}$. Electromagnetic waves vary in wavelength and frequency, and can be grouped based on these features.
The higher the frequency of an electromagnetic wave, the more energy it carries. The human eye can only detect the visible light spectrum of electromagnetic waves, and can differentiate different frequency waves as different colours.
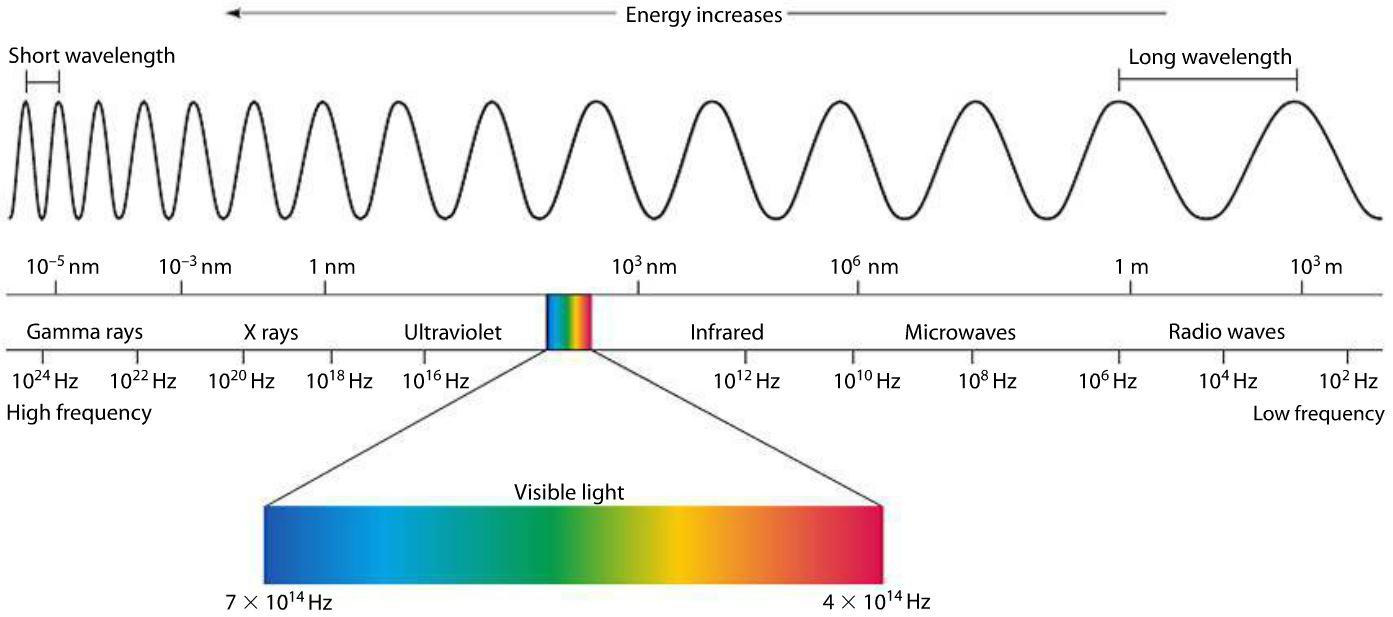
Radio Waves
Radio waves have the longest wavelength and the lowest frequency on the electromagnetic spectrum. This makes them good for communication. Radio waves can be emitted with a transmitter. Transmitters use alternating currents to create a radio wave. This wave will have the same frequency as the current that created it. Radio waves can also cause an alternating current. Which can then be received by a receiver.
-
TV and FM radio use short wavelength radio waves, which can easily be blocked and only work for short distances.
-
MW and LW radio use medium and long wave radio waves, these can travel long distances as they can diffract through the atmosphere and curve around the earth.
-
Bluetooth uses short radio waves to send data between devices.
Microwaves
Microwaves can be used for communication and cooking.
Microwaves that aren’t the same wavelength as water molecules, are used for satellite communication, this is because they can pass through the atmosphere, which contains relatively high concentrations of water vapour.
Microwaves that can be absorbed by water molecules can be used for cooking. As water molecules gain energy, they increase their kinetic energy. This spreads by conduction or convection to the the other molecules of food, which heats the food.
Infra-red Waves
Infra-red radiation is emitted by any object with thermal energy. The amount of radiation an object gives off is highly dependant on the amount of thermal energy it has.
This means that sensors such as infra-red cameras can be used to detect infra-red and process this information into an image. This means that the hotter an object is, the brighter it will appear. This can be especially useful for night vison cameras.
Infra-red can also be used for cooking and heating, for example, toasters and ovens use infra-red radiation to heat and cook food. Furthermore, some electric heaters use infra-red for heating.
Visible light
Visible light is the spectrum of light that humans can see, we perceive different frequencies of light as different colours.
Ultraviolet Waves
Ultraviolet radiation is often used as a feature of florescent chemicals, where ultraviolet radiation is absorbed and then re-emitted as visible light. This makes florescent lights very energy-efficient especially for long periods of time.
Other uses of ultraviolet radiation include security pens and marks that only become visible under UV light, which helps identify property.
Ultraviolet radiation can cause damage to the DNA in our skin cells, which makes it important to wear sunscreen in strong sunlight.
X-Rays
X-Rays are commonly used to view the internal structure of objects and materials, including the human body. They can affect photographic film the same way as visible light, however most X-Rays are taken digitally these days.
Radiographers in hospitals take X-Ray images to help diagnose broken bones. This works as X-rays transmit through flesh, but are absorbed by denser materials like bones or metal. Thus, X-Rays are fired through a body at a detector, which can produce a negative image of the dense materials in the body.
However, too much exposure to X-Rays can be damaging as it is a form of ionising radiation, thus, where possible, lead aprons, shields and other protective equipment is used.
Gamma Rays
P6 - Radioactivity
Radiation
Isotopes
Atoms are made of a nucleus consisting of protons and neutrons surrounded by electrons. The mass number is the number of protons and neutrons in the nucleus and is represented with this notation:
\[\ce{^{A}_{Z}X} \implies \ce{^{12}_{6}C}\]$\ce{X}$ = Chemical symbol for element
$\ce{A}$ = Mass number
$\ce{Z}$ = Atomic number
Isotopes are atoms of the same element that have a different number of neutrons and therefore different nuclear mass.
For example, protium, deuterium and tritium are all isotopes of hydrogen as they have different neutrons numbers but are all the same element.
\[\begin{aligned} &\text{Protium }& \ce{^{1}_{1}H}&,\\[4pt] &\text{Deuterium }& \ce{^{2}_{1}H}&,\\[4pt] &\text{Tritium }& \ce{^{3}_{1}H}& \end{aligned}\]Most elements only have one or two stable isotopes, other isotopes tend to be unstable and radioactive, meaning they release nuclear radiation.
Alpha Radiation
Alpha radiation or alpha decay is a type of radioactive decay in which an atomic nucleus emits alpha particles($\ce{\alpha}$) which are high-energy helium nuclei ($\ce{He^2+}$) consisting of 2 protons and 2 neutrons. Among the different types of radiation, alpha radiation is the highest energy and most ionising. However, it is also the slowest moving due to its high mass.
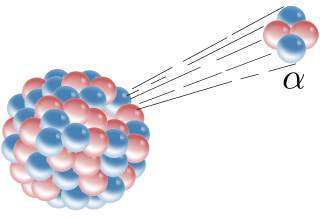
Alpha decay. From: Wikimedia Commons
When an alpha particle is emitted, the atom loses 2 protons and 2 neutrons, this means that both the atomic and mass numbers of the element changes. The change in the number of protons also means that the atom will change elements. This change can be denoted with the following general nuclear equation:
\[\ce{^{A}_{Z}X -> ^{A-4}_{Z-2}X' + ^{4}_{2}\alpha }\]For example, using the general equation, we can write the equation for the alpha decay of radium-226 into radon-222:
\[\ce{^{226}_{88}Ra -> ^{222}_{86}Rn + ^{4}_{2}\alpha }\]Beta Radiation
Beta decay or $\beta$ decay is a type of radioactive decay that occurs when a beta particle is emitted from the nucleus of an atom. A beta particle($\beta$) is a fast and energy high electron($\mathrm{e}^-$).
Beta decay occurs when a neutron transforms into a proton, as a result of the weak nuclear force, this transformation releases an electron($\mathrm{e}^-$) and an electron antineutrino($\overline{\nu}_e$).
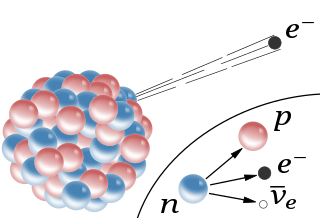
Beta decay. From: Wikimedia Commons
When an atom undergoes beta decay, it gains one of its protons turns into a neutron, this results in a change in atomic number but no change in the overall nuclear mass. The change in atomic number means that the atom will become a different element. Beta decay can be denoted with the following general nuclear equation:
\[\ce{^{A}_{Z}X -> ^{A}_{Z + 1} X' + e^-} + \overline{\nu}_e\]For example, the decay of carbon-14 into nitrogen-14 which has the half-life of about 5,730 years, can be denoted as:
\[\ce{^{14}_{6}C -> ^{14}_{7}N + e^-}+ \overline{\nu}_e\]Gamma Radiation
Gamma radiation is the most penetrating form of radiation and is represented with the symbol $\gamma$, it is the shortest wavelength of electromagnetic waves, typically shorter than those of X-rays.
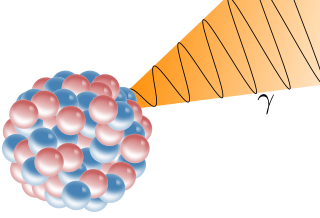
Gamma decay. From: Wikimedia Commons
As gamma rays are massless forms of pure energy, they do not alter the atom on a nuclear level, and so the follow general nuclear equation can be used:
\[\ce{^{A}_{Z}X -> ^{A}_{Z}X }+ \gamma\]For example, protactinium-234 can be denoted as:
\[\ce{^{234}_{91}Pa -> ^{234}_{91}Pa}+ \gamma\]Radiation properties
Different types of nuclear radiation have different types of properties. Radiation with higher penetration can travel through matter for longer before ionising atoms. Radiation with less penetration is typically less ionising as it has a lower chance of interacting with matter therefore granting it lower penetration.
Alpha, beta and gamma radiation are types of ionising radiation, however, they have different ionising power.
| Radiation | Penetrating power | Can be blocked by | Ionising power |
|---|---|---|---|
| Alpha($^{4}_{2}\alpha$) | Low | Piece of paper | High |
| Beta($\beta^-$) | Medium | Thin aluminium | Medium |
| Gamma($\gamma$) | High | Think lead | Low |
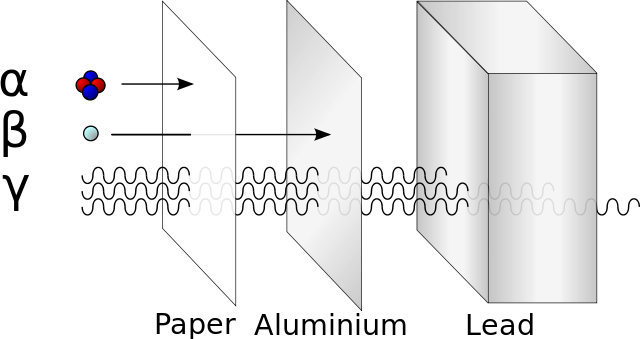
Alpha beta gamma radiation penetration. From: Wikimedia Commons
Emitting Radiation
Electron energy levels
Electrons orbiting an atom sit at different energy levels also known as shells. Electrons in shells that are further away from the nucleus have more energy.
Electrons can absorb EM radiation and move up a shell as they gain energy. Or they can emit EM radiation and move down a shell as they lose energy.
If an atom’s outer electron absorbs enough radiation, it can exit the atom, which ionises the atom. Nuclear radiation can ionise atoms and so is called ionising radiation, ionising radiation can penetrate our cells and cause damage to DNA.
Half-Lives
Unstable and radioactive isotopes decay to become more stable. Radioactive decay is completely random, however, there are overall trends in the decay of radioactive materials.
The half-life of a radioactive material is the time take for the number of radioactive nuclei in a sample to half or the time taken for the number of decays, or activity to halve.
This can be presented as,
\[N(t) = N_0 \bigg(\frac{1}{2}\bigg)^{\frac{t}{^t1/2}}\]$\ce{N(t)}$ = quantity of substance remaining
$\ce{N_0}$ = initial quantity of the substance
$\ce{t}$ = time elapsed
$\ce{^t 1\text{/}2}$ = half life of substance
Dangers and uses of Radiation
Irradiation is the process by which objects are exposed to radiation.
Contamination is the process where radioactive material comes into contact with or is consumed.
Ionising radiation can ionise our DNA which can cause mutation and in rare cases lead to cancer.
Uses of radiation in medicine
Medical Tracers
If certain radioactive isotopes are placed inside the patients bloodstream or digestive system, we can track this radiation passing through the body and check if certain organs are working properly.
Radiotherapy
Large amounts of radiation can kill cells, this is useful for treating cancer. Gamma rays can be fired at cancerous cells in an attempt to kill them. Internal methods like placing a radioactive source inside the body can be used too.
Fusion and Fission
Nuclear Fission
Nuclear fusion is the process of splitting large unstable nuclei into smaller nuclei. This releases large amounts of energy. Nuclear fission is commercially used to generate electrical energy in nuclear power plants.
Nuclear fission is conducted in a power plant using a chain reaction, where fast moving neutrons are fired into large unstable nuclei which split into smaller nuclei and more neutrons, releasing energy in the process. The neutrons released then go on to repeat the process, generating more energy.
However, if this reaction is not controlled, too much energy will be released, like what happens with an atomic bomb. So, in a nuclear power plant, control rods are used to absorb neutrons and slow down the reaction.
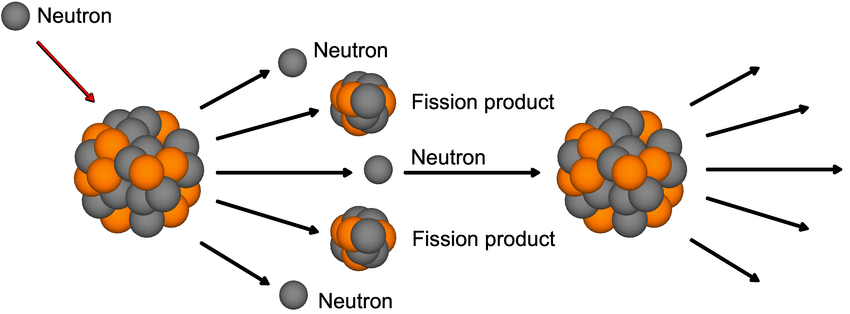
Illustration of nuclear fission. From: Wikimedia Commons
Nuclear fuel is relatively cheap, however, nuclear waste can be problematic and expensive to dispose of.
Nuclear Fusion
Nuclear fusion is the process that occurs in the sun to provide huge amounts of energy. This is done by fusing smaller atoms into larger atoms.
Fusion requires extreme amounts of heat and pressure like that in the core of a star as atoms have to be extremely energetic and close together in order to quantum tunnel into each other.
For example, the fusion of protium and deuterium can be displayed as,
\[\ce{_1^{1}H + _1^{2}H -> _2^3{He} }\]The process of fusion releases energy from mass, and around $0.65\%$ of the mass will be converted into energy $E = mc^2$.
P7 - Energy
Energy is stored in energy stores and this energy can be transferred between or dissipated. For example, a ball rolling up a slope is an example of energy being transferred from its kinetic energy store to its gravitational potential energy store.
However, energy can never be created or destroyed. This means that the total energy of a closed system will never have any net change.
\[K_1 + U_1 = K_2 + U_2\]$K_1$ = initial kinetic energy
$U_1$ = initial potential energy
$K_2$ = final kinetic energy
$U_2$ = final potential energy
Energy Stores and transfer
There are many types of energy stores, such as:
-
Kinetic - energy of an object that is in motion.
-
Thermal - the total kinetic energy of the particles in an object.
-
Chemical - energy stored in the bonds of chemical compounds.
-
Gravitational potential - the potential energy an object posses due to its relation to another massive object due to gravity.
-
Elastic potential - the potential energy stored as a result of applying a force to deform an elastic object.
-
Electromagnetic - two charges/magnets that attract or repel each other.
-
Nuclear - the energy stored in the bonds of the nucleus.
There are four main forms of energy transfer:
- Mechanical - an object moving due to a force.
- Electrically - a charge moving through a potential difference.
- Heating - Energy transferred from a hotter object to a colder object.
- Radiation - Energy transferred by light or sound waves.
Efficiency
Efficiency can be defined as the amount of useful energy output from a system. Efficiency can be calculated by dividing useful energy output by total total input energy.
\[\text{efficiency}=\frac{\text{useful output energy transfer}}{\text{input energy transfer}}\]All real systems have less than $100\%$ energy as factors such as heat, friction etc can all cause a system to lose energy.
Energy lost by heating
Energy can be transferred by heating in three different ways: conduction, convection and radiation.
Conduction
Thermal conduction occurs mainly in solids, and is the process where an the hotter part of an object’s particles transfer their kinetic energy by collision. This continues until heat is spread out along he whole object.
Different objects have different thermal conductivity, which describes how well an object can transfer energy with conduction. For example, metal has a high thermal conductivity while liquids and gases have low thermal conductivity.
Convection
Thermal convection occurs primarily in fluids(liquids and gases). When particles are heated, they move faster and the fluid expands, becoming less dense.
This means that the heated fluid rises above its cooler surroundings. This process will repeat until convection currents appear.
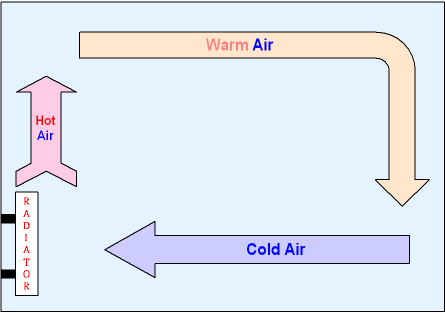
Heat convection. From: GCSE SCIENCE
Radiation
Thermal radiation is the electromagnetic radiation given off by objects with heat. This typically occurs in the infra-red wavelengths.
All objects give off and absorb radiation. Objects colder than their surroundings will absorb more thermal radiation and thus increase their heat, while objects warmer than their surrounds will emit more thermal radiation and thus decrease their heat.
Matt black surfaces are good absorbers and emitters of radiation, light-coloured, smooth and shiny surfaces are poor absorbers of radiation.
Reducing unwanted energy transfers
Insulating buildings
To save energy from heating, many insulation techniques are used on buildings. This saves energy and can make things more efficient.
-
Sealed gaps - All gaps or holes are sealed to prevent air entering or leaving from convection.
-
Cavity walls - Walls with gaps filled with insulating wool in them to reduce conduction as air is a poor conductor.
-
Double glazing - Windows with two layers of glass with an air gap between them to reduce conduction.
-
Thick curtains - Reduce heat loss by convection and conduction through the windows.
Reducing Friction
Friction can be reduced to save energy and increase efficiency. This can be done with lubrication, such as using oil on a bike change. Air resistance can be reduced by making the object streamlined, such as a plane.
P8 - Global Challenges
Everyday motions
Stopping distance and reaction times
Stopping distance is the minimum distance required to stop a vehicle in an emergency. Stopping distance can be calculated by adding breaking distance and thinking distance.
\[\text{stopping distance} = \text{thinking distance}+\text{breaking distance}\]Thinking distance is the amount of distance the car travels in driver’s reaction time. This can be affected by two main factors:
1) Reaction time - If a driver is affected by tiredness, alcohol, distractions or intoxicants, it will take longer for them to notice the hazard and apply brakes.
2) Vehicle speed - the faster a vehicle is travelling, the more distance it will travel during said reaction time.
Breaking distance is the distance taken to stop once breaks have been applied, this can be affected by the mass and speed of vehicle, because $E_k = \frac{1}{2}mv^2$. Other factors that affect breaking distance are:
-
Brake conditions - if the breaks are worn or faulty, they will not be as affective at stopping the car and so the breaking distance will increase.
-
Road conditions and grip - If it is icy or wet, the wheel’s of the vehicle will not have enough grip and so will be more likely to skid and travel a longer distance.
Stopping safely
Speed affects stopping distance a lot. This is because while thinking distance and speed are proportional, speed is proportional to the square of breaking distance. This is because,
\[\textstyle{\frac{1}{2}}mv^2=Fd\]$m$ = mass
$v$ = speed
$F$ = breaking force
$d$ = breaking distance
Energy Sources
Non-Renewable Energy Sources
Most of our energy is from non-renewable sources, meaning that these sources will be depleted one day and can damage the environment. Non-renewable fuels include fossil fuels(coal,oil and natural gas) and nuclear fuels(uranium and plutonium).
Non-renewable fuels such as fossil fuels release $\ce{CO2}$, which contributes to climate change and global warming. Burning coal and oil releases sulphur dioxide, which can cause acid rain.
Oil spillages can cause serious problems for the environment. Nuclear waste is radioactive and difficult to dispose of and nuclear power carries a risk of major catastrophe like the Fukushima disaster in Japan.
Power stations
The most common way of generating electricity is by using steam to drive a turbine. This is usually done by burning fossil fuels.
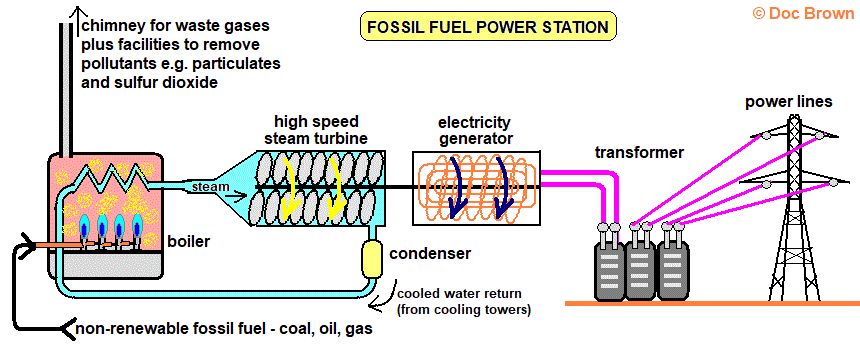
Fossil Fuel Power station. From: ‘Doc Brown’s Chemistry
Nuclear Reactors
A nuclear power station also uses steam to drive a turbine, but the energy comes from nuclear fission instead.
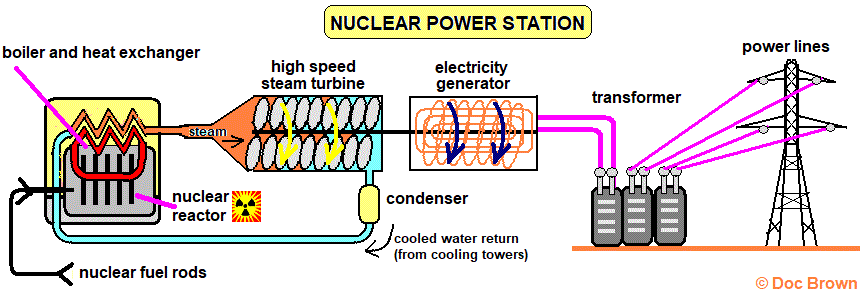
Nuclear Power station. From: ‘Doc Brown’s Chemistry
Renewable Energy Sources
Renewable energy sources cannot be depleted, these include bio-fuels, solar and wind power and hydro power.
Biofuels
Biofuels are made from recently living organisms and includes things like farm waste and algae and plants. Although burning biofuels produce $\ce{CO2}$, this is offset by the fact that they are simply releasing the $\ce{CO2}$ they absorbed. This means that biofuels are considered carbon neutral.
Biofuels can be used the same way as fossil fuels and can be burnt to generate electricity or power cars. Biofuels are cheap to make and can be mixed with fossil fuels like petrol.
However, biofuels need land to grow, which requires space to be made, e.g. clearing forests etc.
Wind and Solar Power
Wind power can be captured through the use of wind turbines, which use wind to turn a generator and create electrical energy for the national grid.
Solar power is usually captured through the use of solar cells, which generate electric current directly through sunlight.
Both of these power sources produce no pollutants and have low running costs. However, they both have high upfront costs.
These power sources are highly dependant on weather conditions and may not be able to keep up with high demand. Furthermore, many people complain that solar cells and wind turbines can be unattractive and noisy.
Hydro Power
Hydro electric dams
Hydroelectric dams trap and store water which are driven through turbines due to gravitational potential energy, this is then converted to electricity.
However, creating a reservoir for the water can lead to loss of natural habitats and damage the ecosystem.
Tidal barrages
Tidal barrages include are big dams build across river estuaries with turbines in them. When the tide comes in, it fill sup the estuary, after this, the dam is closed to create a gravitational potential difference which will generate energy once the water passes through the turbines.
The National Grid
UK domestic electricity is alternating current at $50\mathrm{Hz}$ and $230\mathrm{V}$. The national grid is a network of wires and transformers that connects power stations to consumers.
However, transmitting large amounts of energy at high currents over long distances causes the wire to heat up and lose energy to the surrounds. Because $P = I^2R$, it is more efficient to boost the voltage very high to around $400 000\mathrm{V}$, this keeps the current low and ensures that minimal power is lost.
To get voltage to $400000\mathrm{V}$, step up transformers are used followed by step down transformers to the consumer.
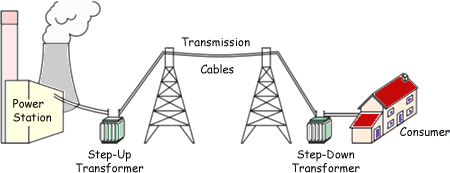
The national grid. From: A Cyberphysics Page
UK Plug
Live Wire
The neutral wire is coated in brown insulation and carries the voltage. It alternates between positive and negative voltage of about $230\mathrm{V}$.
Neutral Wire
The neutral wire is blue and completes the circuit, when the appliance is active, current flows through the live wire into the neutral wire. The neutral wire is around $0\mathrm{V}$.
Earth Wire
The earth wire is yellow/green dosnt normally carry a current, but just in case the live wire comes lose and touches the casing, the earth wire provides an alternative pathway for the current to flow away and not shock or kill a person. The earth wire is around $0\mathrm{V}$.
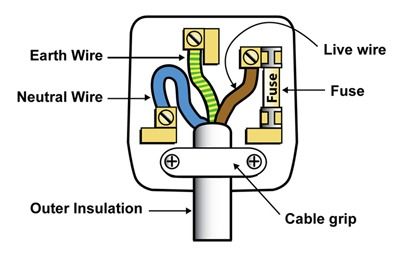
UK Plug Wiring Diagram. From: Pinterest
The Universe
Our Solar System
Our Solar System is made up of one star (the sun) and orbiting structures, which includes,
-
planets - Massive objects that orbit a star, such as Earth.
-
minor planets - Less massive objects that behave like planet’s but do not match the specified criteria to be one.
-
natural satellites - objects that orbit planets, such as the moon.
-
artificial satellites - man-made objects that have been specially designed to orbit a planet.
Orbits
Most orbits are almost circular, which means that the object constantly changes direction, meaning that its velocity is constantly changing, which means that it is accelerating, as $a = \frac{\Delta v}{\Delta t}$.
This is acceleration is caused by gravity, and if the object was did not have enough velocity, it would fall towards the centre of gravity. However, because it already has velocity, it maintains a moment at right angles to the centre of gravity.
A good way to understand orbits is with Newton’s canon thought experiment, varying the amount of initial velocity that the canon ball has changes its flight path until it achieves orbit, however, too much velocity will send it flying into space.
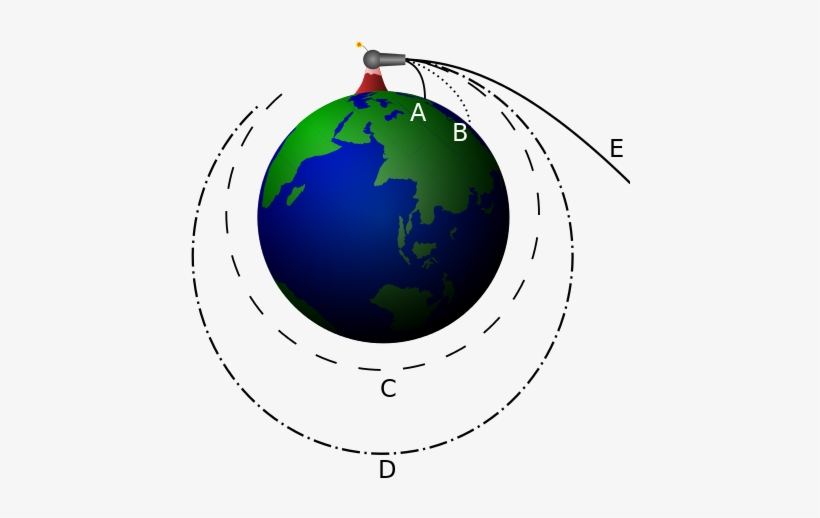
Newton’s Canonball. From: seekpng
This means that because gravitational force increases the closer an object is to its centre, objects must be moving at high speeds in order to orbit close to the centre of gravity, and at slower speeds when orbiting further away.
Artificial satellites
There are two main types of orbits for artificial satellites, geostationary and polar.
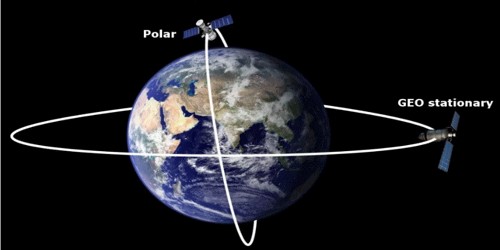
Polar and Geo orbits. From: QS Study
Geostationary Orbit
Satellites in geostationary orbit have a high orbit, as they have to travel relatively slow as they follow the earth’s 24 hour rotation. This means that they stay at the same point above the earth’s surface and rotate along with the earth.
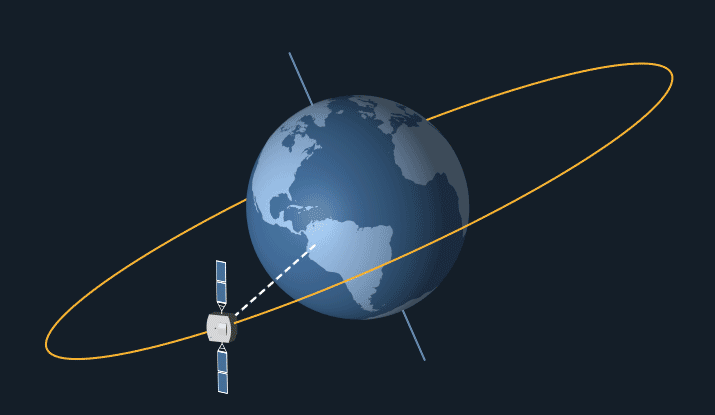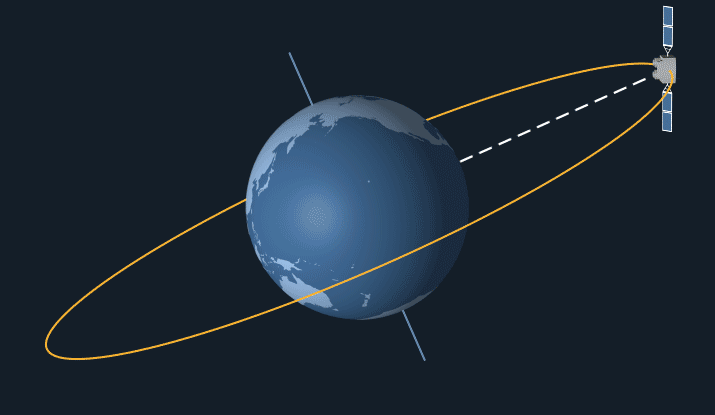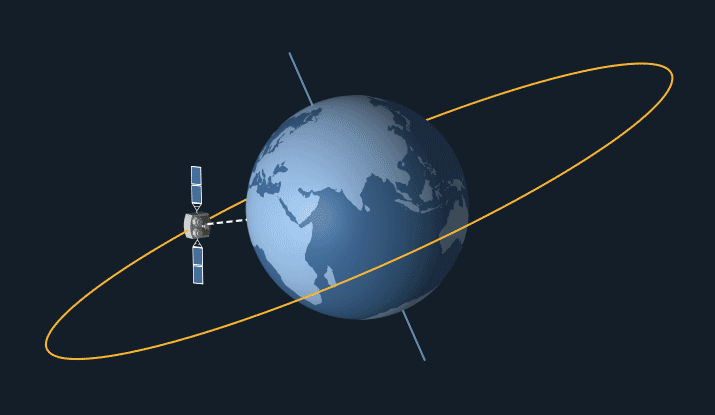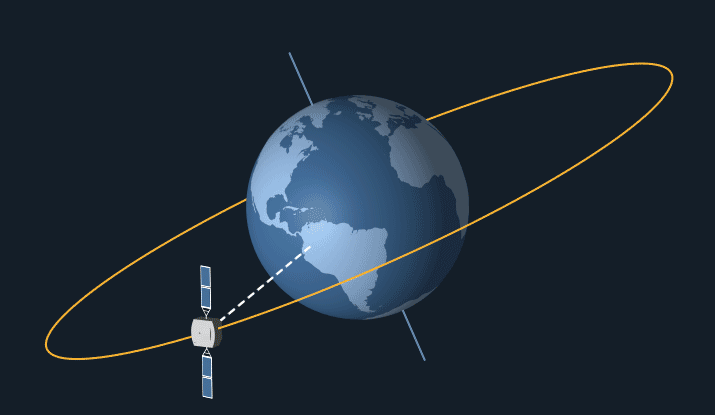
Geostationary orbit. From: NASA Science
Geostationary satellites are ideal for communications such as telephone, TV and radio as they stay at the same point above earth, so it’s easy to locate and pint transmitters at them. Meaning that they can easily transfer signals to other satellites and back down to earth in fractions of a second.
Polar Orbit
Satellites in polar orbit orbit much closer to the surface, and so have to travel significantly faster to stay in orbit. This also means that they complete an orbit much faster, typically in two hours.
Because they have such fast orbits, they can scan the entire earth in a day as the earth rotates. This makes them perfect for weather, mapping and surveillance.

Polar orbit principle. From: EUMETSAT
The Big Bang
The big bang theory is a widely accepted theory that the universe began as a singularity exploding outwards expanding space-time.
Redshift
All stars emit light, however when we look at distant galaxies, the frequencies of light seem to be shifted towards the red end of the spectrum. We call this redshift.
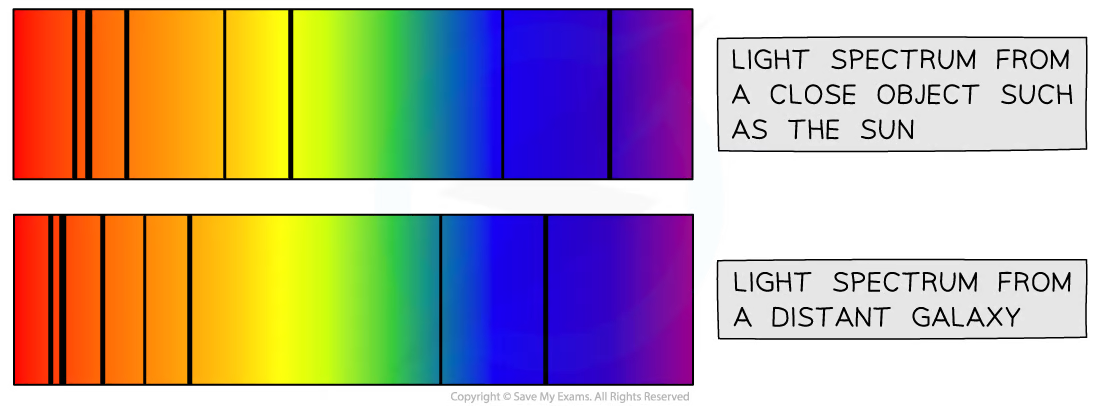
Galactic Redshift. From: Save My Exams
Redshift is a result of the doppler effect, where the wave frequencies emitted from objects moving away relative to the observer are lower, while moving towards an observer are higher.
So, because more distant stars and galaxies are more red-shifted, we can deduce that they are moving away from us faster. This means that if we extrapolate their movements backwards, we can trace all of the galaxies and stars in our universe to a general centralised location.
Cosmic Microwave Background Radiation
CMBR is leftover radiation from the beginning of the universe that shows us the small quantum fluctuations that occurred. This tells us that those interactions that emitted high energy radiation has been stretched out as space-time expanded and is now in the microwave range.
The life cycle of a Star
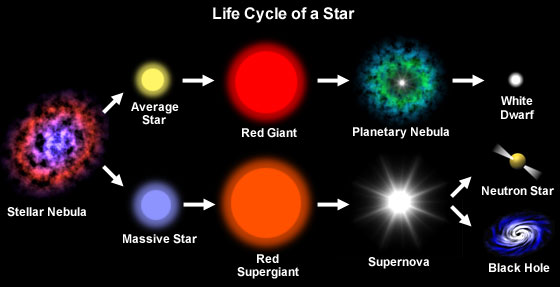
Life Cycle of a Star. From: National Schools’ Observatory
Stellar Nebulae
Stars initially form from clouds of dust and gas(mostly hydrogen) called stellar nebula. These clouds swirl around together due to gravity and eventually clump together to form large blobs.
Protostars
These blobs draw closer and closer due to gravity and eventually clump together in one ball. When the pressure and temperature gets high enough, nuclear fusion occurs to form helium nuclei. The star starts glowing.
Main Sequence Stars
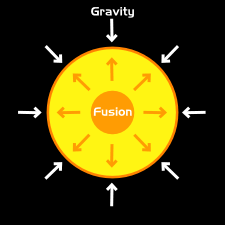
Star equilibrium. From: Aspire
The star immediately enters a state of equilibrium, where the outward fusion energy balances out the inward gravitational energy, keeping the star from expanding/collapsing.
The star fuses hydrogen to helium during this period which lasts several billion years.
Red Giants and Supergiants
Eventually, the hydrogen in the star’s core starts to be depleted, and fusion of heavier elements occurs. The star swells up and turns red(as the surface cools). Small-medium stars are called red giants when they reach this stage.
However, bigger stars are called red supergiants, these stars undergo several stages of nuclear fusion fusing heavier and heavier elements until the star reaches iron.
White dwarfs
Once a small-medium sized star runs out of fuel, it becomes unstable and ejects its outer layer of dust and gas a a planetary nebula. This leaves behind the core of the star, a white dwarf, which slowly cools and becomes a black dwarf once its life span is over.
Neutron Stars and Black Holes
For red supergiants, once they reach iron, fusion suddenly ends and the star collapses in on itself as the equilibrium is broken. This causes a massive explosion known as a supernova.
Neutron Stars
After the supernova, only the very dense core of the supernova remains. Neutron stars are called neutron stars as they are so dense that the electrons and protons fuse together via the weak nuclear force and become neutrons. Effectively making the neutron star a giant nuclei.
It is also possible that the core of a neutron star is composed of quark-gluon plasma, where the conditions are so extreme that neutrons break down into quarks and gluons that move around freely.
Black Hole
For the most massive stars, after the supernova, the core collapses in on itself and becomes a black hole, composed of a singularity or ringularity, an infinitely dense point of space.
Black holes are so dense that beyond the even horizon, not even light can escape, which is why they are perceived as black.
Emitting and Absorbing Radiation
All objects absorb and emit electromagnetic radiation, when an objects absorbs radiation, it becomes hotter.
When an object is hotter than its surroundings, it will emit more radiation than it absorbs, cooling it down. When an object is colder than its surroundings, it will absorb more radiation than it emits, heating it up.
Radiation and the Earth
The sun emits high amounts of electromagnetic radiation, which is absorbed by both the earth and the atmosphere. The overall temperature of the earth depends on how much is emitted and absorbed.
During the day, more radiation is absorbed than is emitted, increasing local temperature. During the night, less radiation is emitted than is absorbed, decreasing local temperature.
However, much of the radiation is absorbed by our atmosphere, especially greenhouse gases such as $\ce{CO2}$. This means that night and day do not have as extreme temperature differences as other planets in our solar system. Furthermore, because the earth is spinning, one side is always exposed to radiation, keeping the overall temperature of the planet constant.
Sonar and Seismic Waves
Sonar
When a wave arrives at a boundary, it can be reflected, refracted or absorbed. By sending and detecting sound waves, we can work out structures of objects that are difficult to observe.
Sonar is used to measure the depth of the seabed or detect structures in the ocean like submarines. Sound waves are fired from a transmitter and detected by a receiver. The time between the input and output can tell us distance, and the amount of wave returned can tell us if there are things absorbing the waves.
Seismic Waves
Earthquakes, volcanos and explosions all product seismic waves, which are sound waves that travel through the earth.
These waves reach boundaries in different parts of the earth and some are reflected, refracted or absorbed. Seismometers can be used to detect these waves from across the world and figure out the internal structure of the earth.
There are two main types of seismic waves, P waves(pressure/primary waves) and S waves(sheer/secondary waves).
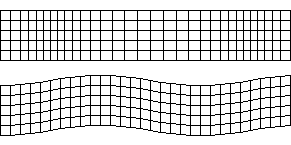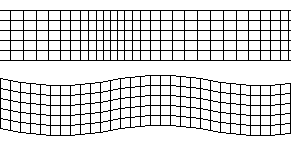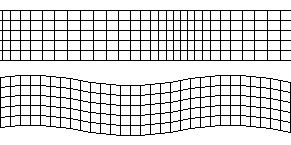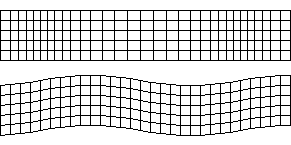
Above: P wave, Below: S wave. From: Wikimedia
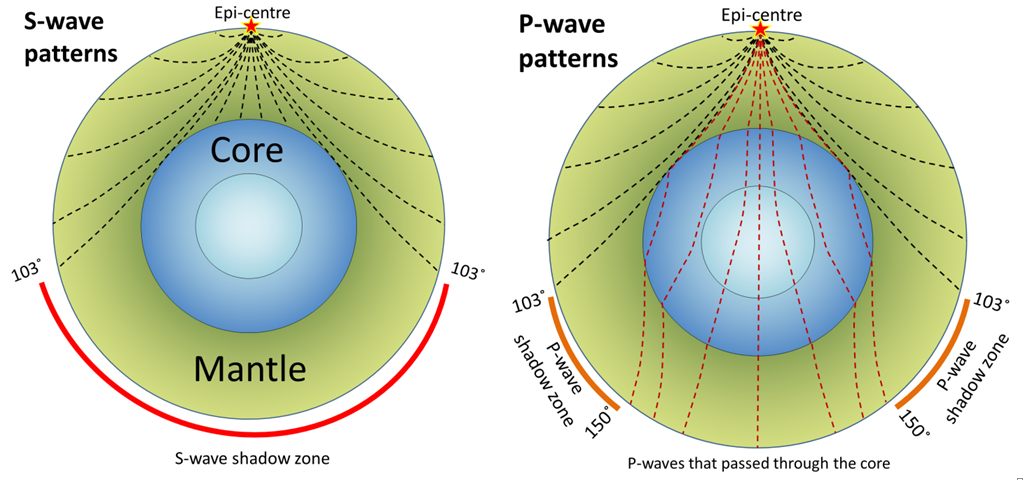
Seismic Waves. From: Digestible Notes
P waves
P waves are longitudinal waves that travel through solids and liquids. They are faster than S waves and hence are called primary waves as they reach seismometers first.
P waves refract sharply at the boundary between the mantel and the core, and so do not reach certain areas at the surface of the earth, these areas are called the shadow zone.
S waves
S waves are transverse waves which cannot travel through liquids. This means that they are absorbed by the liquid outer core, but can travel through the mantle. This causes a large shadow zone, where the waves cannot be detected.
S waves are much slower than P waves.
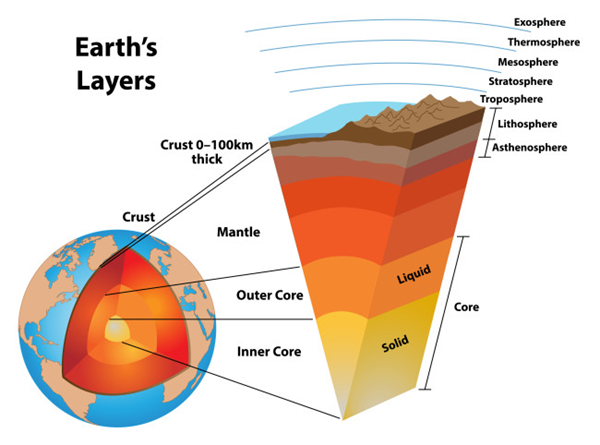
Internal structure of the earth
Our current understanding of the internal structure of the earth is based on these observations.
Earth’s core From: Astronomy Magazine